有机化学 ›› 2022, Vol. 42 ›› Issue (7): 2201-2213.DOI: 10.6023/cjoc202202012 上一篇 下一篇
研究论文
朱雪莉a, 杨绍丽a, 郏彩霞a, 李静b,*( ), 段征a,*(
), 段征a,*( )
)
收稿日期:2022-02-10
修回日期:2022-03-16
发布日期:2022-08-09
通讯作者:
李静, 段征
基金资助:
Xueli Zhua, Shaoli Yanga, Caixia Jiaa, Jing Lib( ), Zheng Duana(
), Zheng Duana( )
)
Received:2022-02-10
Revised:2022-03-16
Published:2022-08-09
Contact:
Jing Li, Zheng Duan
Supported by:文章分享
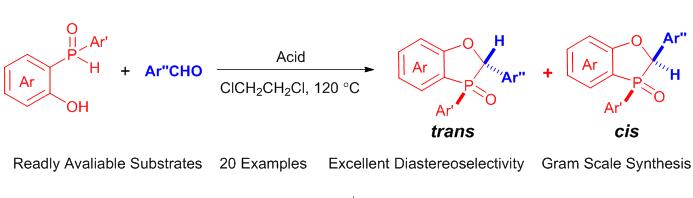
报道了一种利用简单易得的底物, 通过酸促进的缩合环化反应来合成2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯类化合物的新路线. 该反应操作简便, 通过普通硅胶色谱即可实现反应中产生的非对映异构体的分离, 可应用于克级规模合成. 当改用联萘酚磷酸酯[(±)-BINOL-phosphoric acids]为缩合反应促进剂时, 可以实现高非对映立体选择性的构筑苯并氧杂磷杂环戊烯类化合物.
朱雪莉, 杨绍丽, 郏彩霞, 李静, 段征. 通过邻羟基苯基膦和醛的缩合反应合成苯并氧杂磷杂环戊烯[J]. 有机化学, 2022, 42(7): 2201-2213.
Xueli Zhu, Shaoli Yang, Caixia Jia, Jing Li, Zheng Duan. Synthesis of Dihydrobenzooxaphospholes via Cyclocondensation of 2-Phosphinylphenol and Aldehydes[J]. Chinese Journal of Organic Chemistry, 2022, 42(7): 2201-2213.
| Entry | Acid | Solvent | Temp./℃ | Time/h | Yieldb/% |
|---|---|---|---|---|---|
| 1 | p-TSA (2.0 equiv.) | Toluene | 120 | 12 | 45+33 |
| 2 | p-TSA (2.0 equiv.) | MeCN | 120 | 12 | Trace |
| 3 | p-TSA (2.0 equiv.) | 1,2-Dichloroethane | 120 | 12 | 48+39 |
| 4 | p-TSA (2.0 equiv.) | 1,2-Dichloroethane | 100 | 12 | 12+Trace |
| 5 | p-TSA (2.0 equiv.) | 1,2-Dichloroethane | 80 | 12 | Trace |
| 6 | p-TSA (1.0 equiv.) | 1,2-Dichloroethane | 120 | 12 | 9+Trace |
| 7 | p-TSA (10 mol%) | 1,2-Dichloroethane | 120 | 12 | Trace |
| 8 | p-TSA (2.0 equiv.) | 1,2-Dichloroethane | 120 | 8 | 41+29 |
| 9 | CF3COOH (2.0 equiv.) | 1,2-Dichloroethane | 120 | 12 | 56+27 |
| Entry | Acid | Solvent | Temp./℃ | Time/h | Yieldb/% |
|---|---|---|---|---|---|
| 1 | p-TSA (2.0 equiv.) | Toluene | 120 | 12 | 45+33 |
| 2 | p-TSA (2.0 equiv.) | MeCN | 120 | 12 | Trace |
| 3 | p-TSA (2.0 equiv.) | 1,2-Dichloroethane | 120 | 12 | 48+39 |
| 4 | p-TSA (2.0 equiv.) | 1,2-Dichloroethane | 100 | 12 | 12+Trace |
| 5 | p-TSA (2.0 equiv.) | 1,2-Dichloroethane | 80 | 12 | Trace |
| 6 | p-TSA (1.0 equiv.) | 1,2-Dichloroethane | 120 | 12 | 9+Trace |
| 7 | p-TSA (10 mol%) | 1,2-Dichloroethane | 120 | 12 | Trace |
| 8 | p-TSA (2.0 equiv.) | 1,2-Dichloroethane | 120 | 8 | 41+29 |
| 9 | CF3COOH (2.0 equiv.) | 1,2-Dichloroethane | 120 | 12 | 56+27 |


| Entry | Acid | Yieldb/% |
|---|---|---|
| 1 | (rac)-BINOL-phosphoric acid (2.0 equiv.) | 79 |
| 2 | (R)-BINOL-phosphoric acid (2.0 equiv.) | 76 |
| 2 | (S)-BINOL-phosphoric acid (2.0 equiv.) | 62 |
| Entry | Acid | Yieldb/% |
|---|---|---|
| 1 | (rac)-BINOL-phosphoric acid (2.0 equiv.) | 79 |
| 2 | (R)-BINOL-phosphoric acid (2.0 equiv.) | 76 |
| 2 | (S)-BINOL-phosphoric acid (2.0 equiv.) | 62 |
| [1] |
Tang, W.; Qu, B.; Capacci, A. G.; Rodriguez, S.; Wei, X.; Haddad, N.; Narayanan, B.; Ma, S.; Grinberg, N.; Yee, N. K.; Krishnamurthy, D.; Senanayake, C. H. Org. Lett. 2010, 12, 176.
doi: 10.1021/ol9025815 |
| [2] |
(a) Qu, B.; Samankumara, L. P.; Ma, S.; Fandrick, K. R.; Desrosiers, J.-N.; Rodriguez, S.; Li, Z.; Haddad, N.; Han, Z. S.; McKellop, K.; Pennino, S.; Grinberg, N.; Gonnella, N. C.; Song, J. J.; Senanayake, C. H. Angew. Chem., Int. Ed. 2014, 53, 14428.
doi: 10.1002/anie.201408929 pmid: 29896393 |
|
(b) Rodriguez, S.; Qu, B.; Fandrick, K. R.; Buono, F.; Haddad, N.; Xu, Y.; Herbage, M. A.; Zeng, X.; Ma, S.; Grinberg, N.; Lee, H.; Han, Z. S.; Yee, N. K.; Senanayake, C. H. Adv. Synth. Catal. 2014, 356, 301.
doi: 10.1002/adsc.201300727 pmid: 29896393 |
|
|
(c) Qu, B.; Mangunuru, H. P. R.; Wei, X.; Fandrick, K. R.; Desrosiers, J.-N.; Sieber, J. D.; Kurouski, D.; Haddad, N.; Samankumara, L. P.; Lee, H.; Savoie, J.; Ma, S.; Grinberg, N.; Sarvestani, M.; Yee, N. K.; Song, J. J.; Senanayake, C. H. Org. Lett. 2016, 18, 4920.
doi: 10.1021/acs.orglett.6b02401 pmid: 29896393 |
|
|
(d) Handa, S.; Andersson, M. P.; Gallou, F.; Reilly, J.; Lipshutz, B. Angew. Chem., Int. Ed. 2016, 55, 4914.
doi: 10.1002/anie.201510570 pmid: 29896393 |
|
|
(e) Qu, B.; Haddad, N.; Rodriguez, S.; Sieber, J. D.; Desrosiers, J.-N.; Patel, N. D.; Zhang, Y.; Grinberg, N.; Lee, H.; Ma, S.; Ries, U. J.; Yee, N. K.; Senanayake, C. H. J. Org. Chem. 2016, 81, 745.
doi: 10.1021/acs.joc.5b02368 pmid: 29896393 |
|
|
(f) Wei, X.; Qu, B.; Zeng, X.; Savoie, J.; Fandrick, K. R.; Desrosiers, J.-N.; Tcyrulnikov, S.; Marsini, M. A.; Buono, F.; Li, Z.; Yang, B.-S.; Tang, W.; Haddad, N.; Gutierrez, O.; Wang, J.; Lee, H.; Ma, S.; Campbell, S.; Lorenz, J. C.; Eckhardt, M.; Himmelsbach, F.; Peters, S.; Patel, N. D.; Tan, Z.; Yee, N. K.; Song, J. J.; Roschangar, F.; Kozlowski, M. C.; Senanayake, C. H. J. Am. Chem. Soc. 2016, 138, 15473.
doi: 10.1021/jacs.6b09764 pmid: 29896393 |
|
|
(g) Haddad, N.; Mangunuru, H. P. R.; Fandrick, K. R.; Qu, B.; Sieber, J. D.; Rodriguez, S.; Desrosiers, J.-N.; Patel, N. D.; Lee, H.; Kurouski, D.; Grinberg, N.; Yee, N. K.; Song, J. J.; Senanayake, C. H. Adv. Synth. Catal. 2016, 358, 3522.
doi: 10.1002/adsc.201600889 pmid: 29896393 |
|
|
(h) Li, G.; Wang, X.-J.; Zhang, Y.; Tan, Z.; Decroos, P.; Lorenz, J. C.; Wei, X.; Grinberg, N.; Yee, N. K.; Senanayake, C. H. J. Org. Chem. 2017, 82, 5456.
doi: 10.1021/acs.joc.7b00491 pmid: 29896393 |
|
|
(i) Patel, N. D.; Sieber, J. D.; Tcyrulnikov, S.; Simmons, B. J.; Rivalti, D.; Duvvuri, K.; Zhang, Y.; Gao, D. A.; Fandrick, K. R.; Haddad, N.; Lao, K. S.; Mangunuru, H. P. R.; Biswas, S.; Qu, B.; Grinberg, N.; Pennino, S.; Lee, H.; Song, J. J.; Gupton, B. F.; Garg, N. K.; Kozlowski, M. C.; Senanayake, C. H. ACS Catal. 2018, 8, 10190.
doi: 10.1021/acscatal.8b02509 pmid: 29896393 |
|
|
(j) Zatolochnaya, O. V.; Rodriguez, S.; Zhang, Y.; Lao, K. S.; Tcyrulnikov, S.; Li, G.; Wang, X.-J.; Qu, B.; Biswas, S.; Mangunuru, H. P. R.; Rivalti, D.; Sieber, J. D.; Desrosiers, J.-N.; Leung, J. C.; Grinberg, N.; Lee, H.; Haddad, N.; Yee, N. K.; Song, J. J.; Kozlowski, M. C.; Senanayake, C. H. Chem. Sci. 2018, 9, 4505.
doi: 10.1039/c8sc00434j pmid: 29896393 |
|
|
(k) Lu, Z.; Jasinski, J. B.; Handa, S.; Hammond, G. B. Org. Biomol. Chem. 2018, 16, 2748.
doi: 10.1039/C8OB00527C pmid: 29896393 |
|
|
(l) Li, G.; Zatolochnaya, O. V.; Wang, X.-J.; Rodriguez, S.; Qu, B.; Desrosiers, J.-N.; Mangunuru, H. P. R.; Biswas, S.; Rivalti, D.; Karyakarte, S. D.; Sieber, J. D.; Grinberg, N.; Wu, L.; Lee, H.; Haddad, N.; Fandrick, D. R.; Yee, N. K.; Song, J. J.; Senanayake, C. H. Org. Lett. 2018, 20, 1725.
doi: 10.1021/acs.orglett.8b00139 pmid: 29896393 |
|
|
(m) Xu, G.; Senanayake, C. H.; Tang, W. Acc. Chem. Res. 2019, 52, 1101.
doi: 10.1021/acs.accounts.9b00029 pmid: 29896393 |
|
| [3] |
Zhu, J.; Huang, L.; Dong, W.; Li, N.; Yu, X.; Deng, W.-P.; Tang, W. Angew. Chem., Int. Ed. 2019, 58, 16119.
doi: 10.1002/anie.201910008 |
| [4] |
Li, C.; Wan, F.; Chen, Y.; Peng, H.; Tang, W.; Yu, S.; McWilliams, J. C.; Mustakis, J.; Samp, L.; Maguire, R. J. Angew. Chem., Int. Ed. 2019, 58, 13573.
doi: 10.1002/anie.201908089 |
| [5] |
Dong, W.; Xu, X.; Ma, H.; Lei, Y.; Lin, Z.; Zhao, W. J. Am. Chem. Soc. 2021, 143, 10902.
doi: 10.1021/jacs.1c06697 |
| [6] |
Qian, C.; Tang, W. Org. Lett. 2020, 22, 4483.
doi: 10.1021/acs.orglett.0c01490 |
| [7] |
Xiong, W.; Xu, G.; Yu, X.; Tang, W. Organometallics 2019, 38, 4003.
doi: 10.1021/acs.organomet.9b00194 |
| [8] |
(a) Takale, B. S.; Thakore, R. R.; Handa, S.; Gallou, F.; Reilly, J.; Lipshutz, B. H. Chem. Sci. 2019, 10, 8825.
doi: 10.1039/C9SC02528F |
|
(b) Sieber, J. D.; Buono, F.; Brusoe, A.; Desrosiers, J. N.; Haddad, N.; Lorenz, J. C.; Xu, Y.; Wu, H.; Zhang, L.; Han, Z. S. Roschangar, F.; Song, J. J.; Yee, N. K.; Senanayake, C. H. J. Org. Chem. 2019, 84, 4926.
doi: 10.1021/acs.joc.8b03040 |
|
| [9] |
Yang, H.; Sun, J.; Gu, W.; Tang, W. J. Am. Chem. Soc. 2020, 142, 8036.
doi: 10.1021/jacs.0c02686 pmid: 32240585 |
| [10] |
Li, B.; Li, T.; Aliyu, M.; Li, Z. H.; Tang, W. Angew. Chem., Int. Ed. 2019, 58, 11355.
doi: 10.1002/anie.201905174 |
| [11] |
Wu, T.; Kang, X.; Bai, H.; Xiong, W.; Xu, G.; Tang, W. Org. Lett. 2020, 22, 4602.
doi: 10.1021/acs.orglett.0c01129 |
| [12] |
Heinicke, J.; Tzschach, A. Phosphorus Sulfur Relat. Elem. 1985, 25, 345.
|
| [13] |
(a) Li, S.; Han, Z. S.; Viereck, P.; Lee, H.; Kurouski, D.; Senanayake, C. H.; Tsantrizos, Y. S. Org. Lett. 2017, 19, 894.
doi: 10.1021/acs.orglett.7b00051 |
|
(b) Han, Z.S.; Wu, H.; Xu, Y.; Zhang, Y.; Qu, B.; Li, Z.; Caldwell, D. R.; Fandrick, K. R.; Zhang, L.; Roschangar, F.; Song, J.; Senanayake, C. H. Org. Lett. 2017, 19, 1796.
doi: 10.1021/acs.orglett.7b00568 |
|
| [14] |
(a) Heinicke, J.; Tzschach, A. Z. Chem 1980, 20, 342.
doi: 10.1002/zfch.19800200912 pmid: 22899483 |
|
(b) Washington, M. P.; Gudimetla, V. B.; Laughlin, F. L.; Deligonul, N.; He, S.; Payton, J. L.; Simpson, M. C.; Protasiewicz, J. D. J. Am. Chem. Soc. 2010, 132, 4566.
doi: 10.1021/ja1009426 pmid: 22899483 |
|
|
(c) Washington, M. P.; Payton, J. L.; Simpson, M. C.; Protasiewicz, J. D. Organometallics 2011, 30, 1975.
doi: 10.1021/om200014f pmid: 22899483 |
|
|
(d) Laughlin, F. Li; Deligonul, N.; Rheingold, A. L.; Golen, J. A.; Laughlin, B. J.; Smith, R. C.; Protasiewicz, J. D. Organometallics 2013, 32, 7116.
doi: 10.1021/om400838g pmid: 22899483 |
|
|
(e) Wu, S. S.; Rheingold, A. L.; Protasiewicz, J. D. Chem. Commun. 2014, 50, 11036.
doi: 10.1039/C4CC05259E pmid: 22899483 |
|
|
(f) Laughlin, F. L.; Rheingold, A. L.; Deligonul, N.; Laughlin, B. J.; Smith, R. C.; Higham, L. J.; Protasiewicz, J. D. Dalton Trans. 2012, 41, 12016.
pmid: 22899483 |
|
|
(g) Wu, S. S.; Deligonal, N.; Protasiewicz, J. D. Dalton Trans. 2013, 42, 14866.
doi: 10.1039/c3dt51919h pmid: 22899483 |
|
| [15] |
Oehme, H.; Leissring, E. Tetrahedron 1981, 37, 753.
doi: 10.1016/S0040-4020(01)97693-8 |
| [16] |
Huang, H.; Denne, J.; Yang, C.-H.; Wang, H.; Kang, J. Y. Angew. Chem., Int. Ed. 2018, 57, 6624.
doi: 10.1002/anie.201802082 |
| [17] |
(a) Melvin, L. S. Tetrahedron Lett. 1981, 22, 3375.
doi: 10.1016/S0040-4039(01)81909-2 pmid: 29924885 |
|
(b) Heinicke, J.; Böhle, I.; Tzschach, A. J. Organomet. Chem. 1986, 317, 11.
doi: 10.1016/S0022-328X(00)99340-9 pmid: 29924885 |
|
|
(c) Dhawan, B.; Redmore, D. J. Org. Chem. 1991, 56, 833.
doi: 10.1021/jo00002a060 pmid: 29924885 |
|
|
(d) Li, S. S.; Wang, G. Q. Phosphorus Sulfur Relat. Elem. 1991, 61, 119.
doi: 10.1080/10426509108027344 pmid: 29924885 |
|
|
(e) Paladino, J.; Guyard, C.; Thurieau, C.; Fauchère, J. - L. Helv. Chim. Acta 1993, 76, 2465.
doi: 10.1002/hlca.19930760706 pmid: 29924885 |
|
|
(f) Taylor, C. M.; Watson, A. J. Curr. Org. Chem. 2004, 8, 623.
doi: 10.2174/1385272043370717 pmid: 29924885 |
|
|
(g) Whisler, M. C.; MacNeil, S.; Snieckus, V.; Beak, P. Angew. Chem., Int. Ed. 2004, 43, 2206.
doi: 10.1002/anie.200300590 pmid: 29924885 |
|
|
(h) Jayasundera, K. P.; Watson, A. J.; Taylor, C. M. Tetrahedron Lett. 2005, 46, 4311.
doi: 10.1016/j.tetlet.2005.04.104 pmid: 29924885 |
|
|
(i) Yilmaz, E.; Bier, D.; Guillory, X.; Briels, J.; Ruiz-Blanco, Y. B.; Sanchez-Garcia, E.; Ottmann, C.; Kaiser, M. Chem.-Eur. J. 2018, 24, 13807.
doi: 10.1002/chem.201801074 pmid: 29924885 |
|
|
(j) Korb, M.; Lang, H. Chem. Soc. Rev. 2019, 48, 2829.
doi: 10.1039/C8CS00830B pmid: 29924885 |
|
|
(k) Patel, J. J.; Blackburn, T.; Alessi, M.; Sawinski, H.; Snieckus, V. Org. Lett. 2020, 22, 3860.
doi: 10.1021/acs.orglett.0c01123 pmid: 29924885 |
|
| [18] |
(a) Kenny, N. P.; Rajendran, K. V.; Gilheany, D. G. Chem. Commun. 2015, 51, 16561.
doi: 10.1039/C5CC06389B |
|
(b) Huang, H.; Denne, J.; Yang, C. H.; Wang, H.; Kang, J. Y. Angew. Chem., Int. Ed. 2018, 57, 6624.
doi: 10.1002/anie.201802082 |
|
|
(c) Melvin, L. S. Tetrahedron Lett. 1981, 22, 3375.
doi: 10.1016/S0040-4039(01)81909-2 |
|
|
(d) Yang, S. X.; Li, Y. G.; Wang, J. Chin. J. Org. Chem. 1984, 4, 271. (in Chinese)
|
|
|
( 杨石先, 李玉桂, 王坚, 有机化学, 1984, 4, 271.)
|
| [1] | 张俊杰, 徐学涛. (S)-(–)-Xylopinine和(S)-(+)-Laudanosine的不对称合成[J]. 有机化学, 2023, 43(9): 3297-3303. |
| [2] | 方思强, 刘赞娇, 王天利. Atherton-Todd反应的研究进展[J]. 有机化学, 2023, 43(3): 1069-1083. |
| [3] | 庞丽萍, 杨昌杰, 林洪敏, 李心宇, 唐海涛, 潘英明. 多孔有机膦配体聚合物作为可回收配体用于异吲哚啉酮类化合物的合成[J]. 有机化学, 2022, 42(7): 2117-2123. |
| [4] | 高娜, 初晓辉, 刘洋, 李家柱, 王进军. 焦脱镁叶绿酸的区域和立体选择性的芳(芳酰)亚甲基化及其叶绿素类二氢卟吩衍生物的合成[J]. 有机化学, 2022, 42(4): 1111-1122. |
| [5] | 肖剑, 武志英, 陈姿依, 赵朋飞, 刘春艳. 四乙烯五胺功能化酚醛树脂作为Knoevenagel缩合反应的高活性酸碱双功能催化剂[J]. 有机化学, 2022, 42(4): 1179-1187. |
| [6] | 焦林郁, 于华, 宁资慧, 李卓. 芳基、烷基磷酸混酯类化合物的合成方法研究进展[J]. 有机化学, 2021, 41(11): 4180-4191. |
| [7] | 王凌锋, 钱鹰. 近红外I区喹啉-氟硼二吡咯荧光染料的合成及其在溶酶体内的荧光成像[J]. 有机化学, 2020, 40(5): 1246-1250. |
| [8] | 丁邦东, 姜业朝, 张瑜, 叶蓉, 孙晶, 颜朝国. 喹啉季铵盐和1,3-茚满二酮及2-芳亚甲基1,3-茚满二酮的环加成反应合成茚满酮类含氮杂环化合物[J]. 有机化学, 2020, 40(4): 1003-1016. |
| [9] | 黄远婷, 陈迁. 芳炔参与的磷和硫芳基化反应研究进展[J]. 有机化学, 2020, 40(12): 4087-4100. |
| [10] | 马蓉, 宋格格, 奚秋贞, 杨柳, 李二庆, 段征. 有机磷催化γ-甲基联烯酸酯[3+2]环化反应[J]. 有机化学, 2019, 39(8): 2196-2202. |
| [11] | 黄海洋, 丁海新, 徐双双, 柏江, 肖强. 膦烯化合物的合成及应用研究进展[J]. 有机化学, 2019, 39(5): 1263-1276. |
| [12] | 刘双, 李玉明, 王典, 魏榕, 苗志伟. 手性诱导构建磷手性中心不对称合成有机磷功能化合物研究进展[J]. 有机化学, 2018, 38(2): 341-349. |
| [13] | 张平竹, 王晓芬, 郑雪阳, 连平平, 魏超, 李小六. 氮杂环并二氢嘧啶及嘧啶衍生物的合成及生物活性评价[J]. 有机化学, 2018, 38(12): 3278-3285. |
| [14] | 王群, 江黎黎, 卢斌, 胡北, 谢小敏, 李泽标, 张兆国. 手性辅基诱导β-脱氢氨基酸酯的非均相不对称氢化(英文)[J]. 有机化学, 2017, 37(6): 1398-1406. |
| [15] | 杨佳, 肖晶, 周永波, 陈铁桥, 尹双凤, 韩立彪. 简单易得试剂与磷-氢化合物交叉偶联合成有机磷化合物[J]. 有机化学, 2017, 37(5): 1055-1068. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||