有机化学 ›› 2023, Vol. 43 ›› Issue (3): 1069-1083.DOI: 10.6023/cjoc202210032 上一篇 下一篇
所属专题: 中国女科学家专辑
综述与进展
收稿日期:2022-10-26
修回日期:2022-12-05
发布日期:2022-12-21
通讯作者:
王天利
基金资助:
Siqiang Fanga, Zanjiao Liua, Tianli Wanga,b( )
)
Received:2022-10-26
Revised:2022-12-05
Published:2022-12-21
Contact:
Tianli Wang
Supported by:文章分享

磷酰胺或磷酸酯类化合物及其衍生物是一类非常重要的含磷有机分子, 在药物化学、材料化学以及有机催化等研究领域均有着广泛应用. Atherton-Todd反应是制备这类化合物最有效的方法之一, 该反应是指氧磷氢化合物[P(O)- H]在碱的作用下与四氯化碳原位生成磷酰氯[P(O)-Cl]中间体, 随后该中间体与醇或胺类化合物反应形成相应的磷酸酯或磷酰胺类化合物及其衍生物. 近年来, Atherton-Todd反应由于其操作容易、原子经济性高、底物普适性广以及易于将含磷原子引入到活性化合物结构片段中等优点, 受到了合成化学家的广泛关注. 总结近几十年来Atherton-Todd反应的研究进展及其在有机合成中的应用, 并对目前该领域可能存在的研究挑战提出展望, 希望能为Atherton-Todd反应的未来发展方向提供一些借鉴和思考.
方思强, 刘赞娇, 王天利. Atherton-Todd反应的研究进展[J]. 有机化学, 2023, 43(3): 1069-1083.
Siqiang Fang, Zanjiao Liu, Tianli Wang. Recent Advances of the Atherton-Todd Reaction[J]. Chinese Journal of Organic Chemistry, 2023, 43(3): 1069-1083.
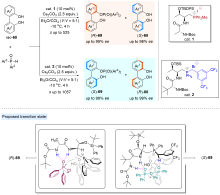
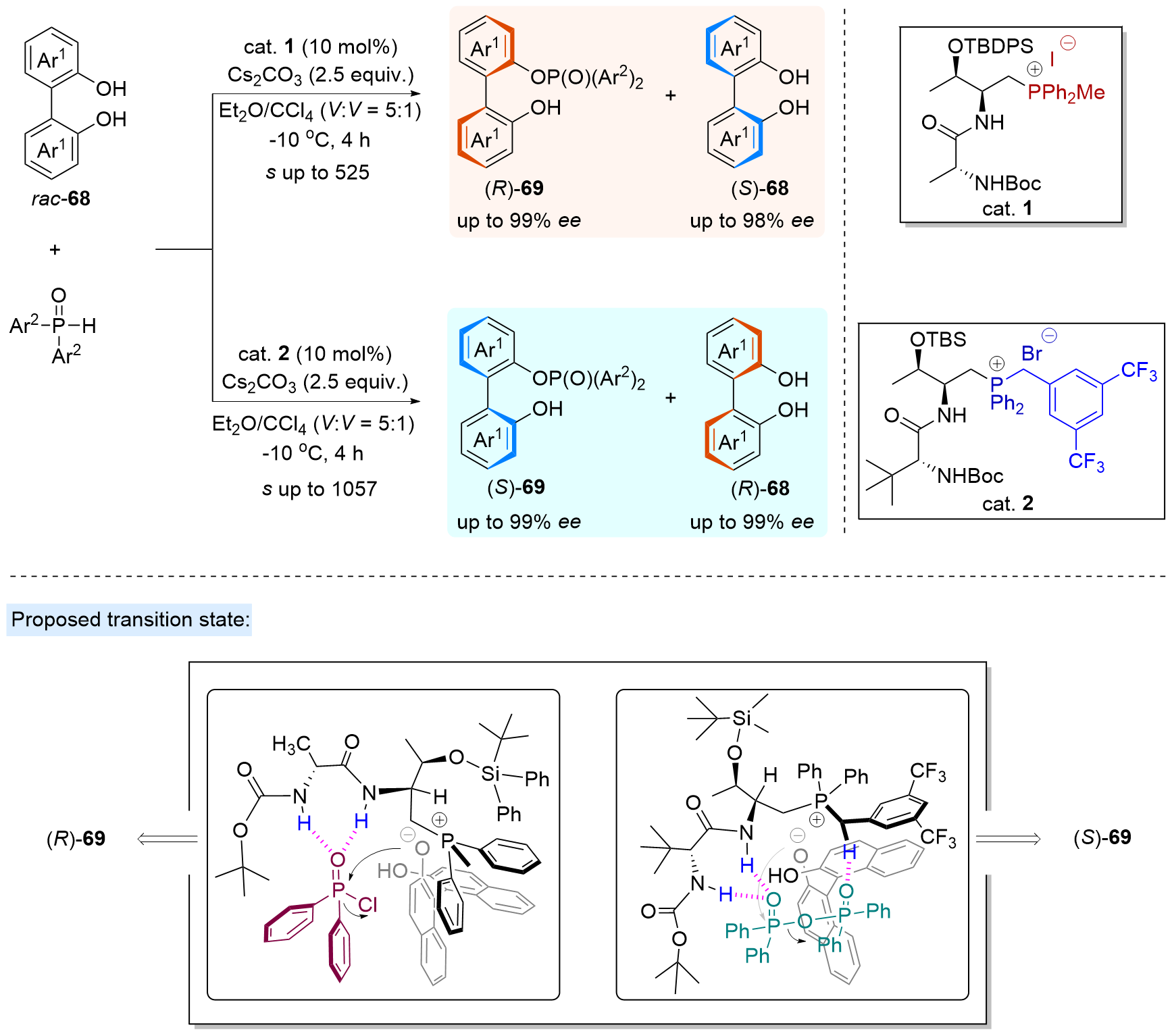
| [1] |
Kannan, P.; Kishore, K. Polymer 1992, 33, 418.
doi: 10.1016/0032-3861(92)91002-J |
| [2] |
Orsini, F.; Sello, G.; Sisti, M. Curr. Med. Chem. 2010, 17, 264.
pmid: 20214568 |
| [3] |
Joachimiak, Ł.; Błażewska, K. M. J. Med. Chem. 2018, 61, 8536.
doi: 10.1021/acs.jmedchem.8b00249 pmid: 29771523 |
| [4] |
Tang, W.; Zhang, X. Chem. Rev. 2003, 103, 3029.
doi: 10.1021/cr020049i |
| [5] |
Dutartre, M.; Bayardon, J.; Jugé, S. Chem. Soc. Rev. 2016, 45, 5771.
pmid: 27479243 |
| [6] |
Kiss, N. Z.; Keglevich, G. Curr. Org. Chem. 2014, 18, 2673.
doi: 10.2174/1385272819666140829011741 |
| [7] |
Atherton, F. R.; Openshaw, H. T.; Todd, A. R. J. Chem. Soc. 1945, 660.
|
| [8] |
Atherton, F. R.; Todd, A. R. J. Chem. Soc. 1947, 674.
|
| [9] |
Cao, S.; Zhao, Y. Sci. China: Chem. 2015, 45, 283.
|
| [10] |
Le Corre, S. S.; Berchel, M.; Couthon-Gourvès, H.; Haelters, J.-P.; Jaffrès. P.-A. Beilstein J. Org. Chem. 2014, 10, 1166.
doi: 10.3762/bjoc.10.117 |
| [11] |
Georgiev, E.; Roundhill, D. M.; Troev, K. Inorg. Chem. 1992, 31, 1965.
doi: 10.1021/ic00036a047 |
| [12] |
Steinberg, G. M. J. Org. Chem. 1950, 637.
|
| [13] |
Krutikov, V. I.; Erkin, A. V.; Krutikova, V. V. Russ. J. Gen. Chem. 2012, 82, 822.
doi: 10.1134/S1070363212050039 |
| [14] |
Kong, A.; Engel, R. Bull. Chem. Soc. Jpn. 1985, 58, 3671.
doi: 10.1246/bcsj.58.3671 |
| [15] |
Troev, K.; Kirilov, E. M. G.; Roundhill, D. M. Bull. Chem. Soc. Jpn. 1990, 63, 1284.
doi: 10.1246/bcsj.63.1284 |
| [16] |
Georgiev, E. M.; Kaneti, J.; Troev, K.; Roundhill, D. M. J. Am. Chem. Soc. 1993, 115, 10964.
doi: 10.1021/ja00076a063 |
| [17] |
Liu, L.; Li, G.; Zeng, X.; Fu, L.; Cao, R. Heteroat. Chem. 1996, 7, 131.
doi: 10.1002/(ISSN)1098-1071 |
| [18] |
Ashmus, R. A.; Lowary, T. L. Org. Lett. 2014, 16, 2518.
doi: 10.1021/ol500894k pmid: 24783964 |
| [19] |
Tan, Y.; Han, Y.-P.; Zhang, Y.; Zhang, H.-Y.; Zhao, J.; Yang, S.-D. J. Org. Chem. 2022, 87, 3254.
doi: 10.1021/acs.joc.1c02933 |
| [20] |
Brands, K. M. J.; Wiedbrauk, K.; Williams, J. M.; Dolling, U.-H.; Reider, P. J. Tetrahedron Lett 1998, 39, 9583.
doi: 10.1016/S0040-4039(98)02300-4 |
| [21] |
Dhineshkumar, J.; Prabhu, K. R. Org. Lett. 2013, 15, 6062.
doi: 10.1021/ol402956b pmid: 24219013 |
| [22] |
Dar, B. A.; Dangroo, N. A.; Gupta, A.; Wali, A.; Khuroo, M. A.; Vishwakarma, R. A.; Singh, B. Tetrahedron Lett 2014, 55, 1544.
doi: 10.1016/j.tetlet.2014.01.064 |
| [23] |
Anitha, T.; Ashalu, K. C.; Sandeep, M.; Mohd, A.; Wencel-Delord, J.; Colobert, F.; Reddy, K. R. Eur. J. Org. Chem. 2019, 2019, 7463.
doi: 10.1002/ejoc.201901362 |
| [24] |
Wang, X.; Ou, Y.; Peng, Z.; Yu, G.; Huang, Y.; Li, X.; Huo, Y.; Chen, Q. J. Org. Chem. 2019, 84, 14949.
doi: 10.1021/acs.joc.9b02301 |
| [25] |
Tan, C.; Liu, X.; Jia, H.; Zhao, X.; Chen, J.; Wang, Z.; Tan, J. Chem.-Eur. J. 2020, 26, 881.
doi: 10.1002/chem.201904237 pmid: 31625634 |
| [26] |
Chen, Q.; Zeng, J.; Yan, X.; Huang, Y.; Wen, C.; Liu, X.; Zhang, K. J. Org. Chem. 2016, 81, 10043.
doi: 10.1021/acs.joc.6b01932 |
| [27] |
Kaboudin, B.; Donyavi, A.; Kazemi, F. Synthesis 2018, 50, 170.
doi: 10.1055/s-0036-1589111 |
| [28] |
Wang, Y.; Qian, P.; Su, J.-H.; Li, Y.; Bi, M.; Zha, Z.; Wang, Z. Green Chem. 2017, 19, 4769.
doi: 10.1039/C7GC01989K |
| [29] |
Li, Q.-Y.; Swaroop, T. R.; Hou, C.; Wang, Z.-Q. Pan, Y.-M.; Tang, H.-T. Adv. Synth. Catal. 2019, 361, 1761.
doi: 10.1002/adsc.v361.8 |
| [30] |
Deng, L.; Wang, Y.; Mei, H. Pan, Y.; Han, J. J. Org. Chem. 2019, 84, 949.
doi: 10.1021/acs.joc.8b02882 |
| [31] |
Dong, X.; Wang, R.; Jin, W.; Liu, C. Org. Lett. 2020, 22, 3062.
doi: 10.1021/acs.orglett.0c00814 |
| [32] |
Li, Y.; Yang, Q.; Yang, L.; Lei, N.; Zheng, K. Chem. Commun. 2019, 55, 4981.
doi: 10.1039/C9CC01378D |
| [33] |
Wang, P.; Tang, S.; Huang, P.; Lei, A. Angew. Chem., Int. Ed. 2017, 56, 3009.
doi: 10.1002/anie.201700012 pmid: 28177563 |
| [34] |
Huang, P.; Wang, P.; Tang, S.; Fu, Z.; Lei, A. Angew. Chem., Int. Ed. 2018, 57, 8115.
doi: 10.1002/anie.v57.27 |
| [35] |
Li, C.-Y.; Liu, Y.-C.; Li, Y.-X.; Reddy, D. M.; Lee, C.-F. Org. Lett. 2019, 21, 7833.
doi: 10.1021/acs.orglett.9b02825 |
| [36] |
Jin, X.; Yamaguchi, K.; Mizuno, N. Org. Lett. 2013, 15, 418.
doi: 10.1021/ol303420g |
| [37] |
Fraser, J.; Wilson, L. J.; Blundell, R. K.; Hayes, C. J. Chem. Commun. 2013, 49, 8919.
doi: 10.1039/c3cc45680c |
| [38] |
Zhou, Y.; Yin, S.; Gao, Y.; Zhao, Y.; Goto, M.; Han L.-B. Angew. Chem., Int. Ed. 2010, 49, 6852.
doi: 10.1002/anie.201003484 |
| [39] |
Li, C.; Chen, T.; Han, L.-B. Dalton Trans. 2016, 45, 14893.
doi: 10.1039/C6DT02236G |
| [40] |
Zhu, Y.; Chen, T.; Li, S.; Shimada, S.; Han, L.-B. J. Am. Chem. Soc. 2016, 138, 5825.
doi: 10.1021/jacs.6b03112 |
| [41] |
Xue, J.-W.; Zeng, M.; Zhang, S.; Chen, Z.; Yin, G. J. Org. Chem. 2019, 84, 4179.
doi: 10.1021/acs.joc.9b00194 |
| [42] |
Zhang, H.; Zhan, Z.; Lin, Y.; Shi, Y.; Li, G.; Wang, Q.; Deng, Y.; Hai, L.; Wu, Y. Org. Chem. Front. 2018, 5, 1416.
doi: 10.1039/C7QO01082F |
| [43] |
Pitre, S. P.; McTiernan, C. D.; Ismaili, H.; Scaiano, J. C. J. Am. Chem. Soc. 2013, 135, 13286.
doi: 10.1021/ja406311g |
| [44] |
Sun, J.-G.; Yang, H.; Li, P.; Zhang, B. Org. Lett. 2016, 18, 5114.
doi: 10.1021/acs.orglett.6b02563 |
| [45] |
Fu, Y.; Duan, F.; Du, Z. Asian J. Org. Chem. 2021, 10, 1071.
doi: 10.1002/ajoc.v10.5 |
| [46] |
Song, S.; Zhang, Y.; Yeerlan, A.; Zhu, B.; Liu, J.; Jiao, N. Angew. Chem., Int. Ed. 2017, 56, 2487.
doi: 10.1002/anie.201612190 pmid: 28112850 |
| [47] |
Ou, Y.; Huang, Y.; He, Z.; Yu, G.; Huo, Y.; Li, X.; Gao, Y.; Chen, Q. Chem. Commun. 2020, 56, 1357.
doi: 10.1039/C9CC09407E |
| [48] |
Handoko.; Benslimane, Z.; Arora, P. S. Org. Lett. 2020, 22, 5811.
doi: 10.1021/acs.orglett.0c01858 pmid: 32672974 |
| [49] |
Yu, X.; Zhang, S.; Jiang, Z.; Zhang, H.-S.; Wang, T. Eur. J. Org. Chem. 2020, 3110.
|
| [50] |
Reiff, L. P.; Aaron, H. S. J. Am. Chem. Soc. 1970, 92, 5275.
doi: 10.1021/ja00720a077 |
| [51] |
Stec, W.; Mikolajczyk, M. Tetrahedron 1973, 29, 539.
doi: 10.1016/0040-4020(73)80006-7 |
| [52] |
Wang, G.; Shen, R.; Xu, Q.; Goto, M.; Zhao, Y.; Han, L-B. J. Org. Chem. 2010, 75, 3890.
doi: 10.1021/jo100473s |
| [53] |
Zhou, Y.; Wang, G.; Saga, Y.; Shen, R.; Goto, M.; Zhao, Y.; Han, L.-B. J. Org. Chem. 2010, 75, 7924.
doi: 10.1021/jo101540d |
| [54] |
Xiong, B.; Zhou, Y.; Zhao, C.; Goto, M.; Yin, S.-F.; Han, L.-B. Tetrahedron 2013, 69, 9373.
doi: 10.1016/j.tet.2013.09.001 |
| [55] |
Cao, S.; Guo, Y.; Wang, J.; Qi, L.; Gao, P.; Zhao, H.; Zhao, Y. Tetrahedron Lett 2012, 53, 6302.
doi: 10.1016/j.tetlet.2012.09.056 |
| [56] |
Cao, S.; Gao P, Guo, Y.; Zhao, H.; Wang, J.; Liu, Y.; Zhao, Y. J. Org. Chem. 2013, 78, 11283.
doi: 10.1021/jo4018342 |
| [57] |
Kenner, G. W.; Williams, N. R. J. Chem. Soc. 1955, 522.
|
| [58] |
Lu, D.; Meng, Z.; Thakur, G. A.; Fan, P.; Steed, J.; Tartal, C. L.; Hurst, D. P.; Reggio, P. H.; Deschamps, J. R.; Parrish, D. A.; George, C.; Järbe, T. U. C.; Lamb, R. J.; Makriyannis, A. J. Med. Chem. 2005, 48, 4576.
doi: 10.1021/jm058175c |
| [59] |
Chopa, A. B.; Lockhart, M. T.; Dorn, V. B. Organometallics 2002, 21, 1425.
doi: 10.1021/om010878e |
| [60] |
Carsten, B.; He, F.; Son, H. J.; Xu, T.; Yu, L. Chem. Rev. 2011, 111, 1493.
doi: 10.1021/cr100320w |
| [61] |
Chen, H.; Huang, Z.; Hu, X.; Tang, G.; Xu, P.; Zhao, Y.; Cheng, C.-H. J. Org. Chem. 2011, 76, 2338.
doi: 10.1021/jo2000034 pmid: 21388215 |
| [62] |
Dutartre, M.; Bayardon, J.; Juge, S. Chem. Soc. Rev. 2016, 45, 5771.
pmid: 27479243 |
| [63] |
Ni, H.; Chan, W.-L.; Lu, Y. Chem. Rev. 2018, 118, 9344.
doi: 10.1021/acs.chemrev.8b00261 |
| [64] |
Melvin, L. S. Tetrahedron Lett. 1981, 22, 3375.
doi: 10.1016/S0040-4039(01)81909-2 |
| [65] |
Ellman, J. A.; Owens, T. D.; Tang, T. P. Acc. Chem. Res. 2002, 35, 984.
doi: 10.1021/ar020066u |
| [66] |
Mohd, A.; Anitha, T.; Reddy, K. R.; Wencel-Delord, J.; Colobert, F. Eur. J. Org. Chem. 2019, 7836.
|
| [67] |
Gatineau, D.; Nguyen, D. H.; Hérault, D.; Vanthuyne, N.; Leclaire, J.; Giordano, L.; Buono, G. J. Org. Chem. 2015, 80, 4132.
doi: 10.1021/acs.joc.5b00548 pmid: 25806668 |
| [68] |
Fang, S.; Tan, J.-P.; Pan, J.; Zhang, H.; Chen, Y.; Ren, X.; Wang, T. Angew. Chem., Int. Ed. 2021, 60, 14921.
doi: 10.1002/anie.v60.27 |
| [69] |
Mäder, P.; Kattner, L. J. Med. Chem. 2020, 63, 14243.
doi: 10.1021/acs.jmedchem.0c00960 |
| [70] |
Otocka, S.; Kwiatkowska, M.; Madalińska, L.; Kiełbasiński, P. Chem. Rev. 2017, 117, 4147.
doi: 10.1021/acs.chemrev.6b00517 |
| [71] |
Fang, S.; Liu, Z.; Zhang, H.; Pan, J.; Chen, Y.; Ren, X.; Wang, T. ACS Catal. 2021, 11, 13902.
doi: 10.1021/acscatal.1c03966 |
| [1] | 杨爽, 房新强. 氮杂环卡宾催化实现的动力学拆分近期研究进展[J]. 有机化学, 2024, 44(2): 448-480. |
| [2] | 陈宛婷, 钟雄威, 邢佳乐, 吴昌书, 高杨. C—N轴手性化合物的不对称催化合成研究进展[J]. 有机化学, 2024, 44(2): 349-377. |
| [3] | 姜权彬. 经由氮杂邻联烯醌中间体合成轴手性化合物的研究进展[J]. 有机化学, 2024, 44(1): 159-172. |
| [4] | 程春霞, 吴露平, 沙风, 伍新燕. 手性叔膦-酰胺不对称催化香豆素与Morita-Baylis-Hillman碳酸酯之间的插烯烯丙基烷基化反应[J]. 有机化学, 2023, 43(9): 3188-3195. |
| [5] | 罗诚, 尹艳丽, 江智勇. P-手性膦氧化物的不对称合成研究进展[J]. 有机化学, 2023, 43(6): 1963-1976. |
| [6] | 王海清, 杨爽, 张宇辰, 石枫. 邻羟基苄醇参与的催化不对称反应研究进展[J]. 有机化学, 2023, 43(3): 974-999. |
| [7] | 曹伟地, 刘小华. 不对称催化质子化构建α-叔碳羰基化合物研究进展[J]. 有机化学, 2023, 43(3): 961-973. |
| [8] | 曾燕, 叶飞. 不对称催化构建硅立体中心化合物的新反应体系研究进展[J]. 有机化学, 2023, 43(10): 3388-3413. |
| [9] | 赵佳怡, 葛怡聪, 何川. 不对称催化Si—H/X—H脱氢偶联构筑硅中心手性[J]. 有机化学, 2023, 43(10): 3352-3366. |
| [10] | 代增进, 张绪穆, 殷勤. 铵盐为胺源的不对称还原胺化反应研究进展[J]. 有机化学, 2022, 42(8): 2261-2274. |
| [11] | 李晖, 殷亮. 铜催化的直接型插烯反应研究进展[J]. 有机化学, 2022, 42(6): 1573-1585. |
| [12] | 吴逾诸, 申盼盼, 段文增, 马玉道. 卡宾催化对亚甲基苯醌的不对称硼化反应的研究[J]. 有机化学, 2022, 42(5): 1483-1492. |
| [13] | 徐萌萌, 蔡泉. 2-吡喃酮的催化不对称Diels-Alder反应研究进展[J]. 有机化学, 2022, 42(3): 698-713. |
| [14] | 陈运荣, 刘炜, 杨晓瑜. 叔醇的动力学拆分研究进展[J]. 有机化学, 2022, 42(3): 679-697. |
| [15] | 闫辉, 张曼, 李琳, 胡腾, 杨武林. 手性螺环缩酮化合物的不对称催化合成研究进展[J]. 有机化学, 2022, 42(11): 3640-3657. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||