有机化学 ›› 2023, Vol. 43 ›› Issue (11): 3989-3996.DOI: 10.6023/cjoc202304013 上一篇 下一篇
研究论文
收稿日期:2023-04-10
修回日期:2023-06-05
发布日期:2023-06-26
基金资助:
Binghao Huo( ), Conghui Guo, Zhanhui Xu(
), Conghui Guo, Zhanhui Xu( )
)
Received:2023-04-10
Revised:2023-06-05
Published:2023-06-26
Contact:
E-mail: Supported by:文章分享
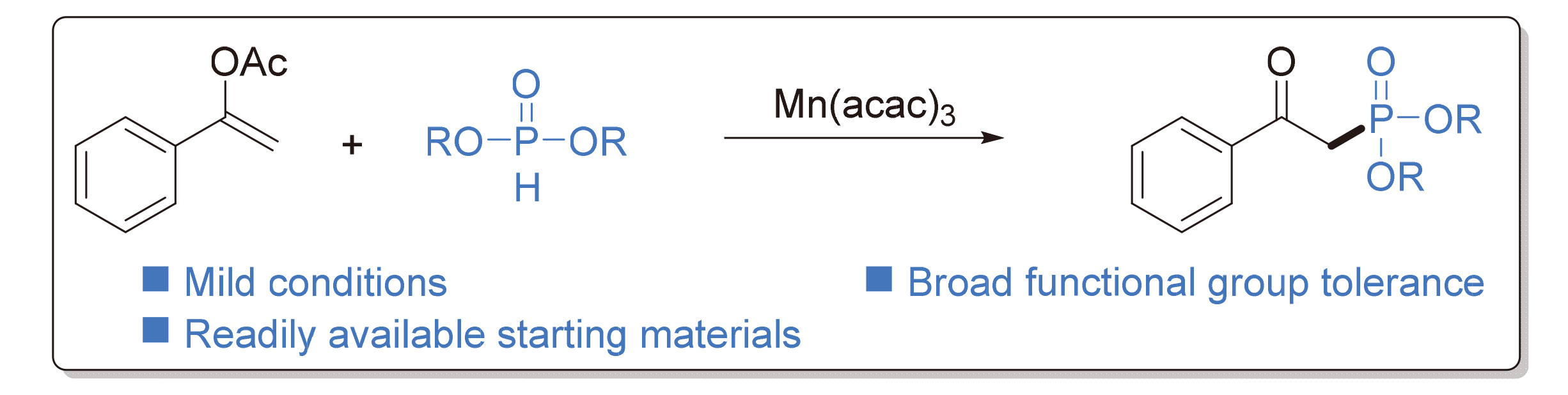
β-酮膦酸酯作为在天然产物和生物活性分子中广泛存在的结构骨架, 在药物化学和有机转化等领域具有重要的合成价值. 报道了一种Mn(acac)3促进的芳基烯醇醋酸酯和烷基亚磷酸酯的氧化偶联反应, 在温和且简单的条件下, 实现了具有广泛官能团兼容性的β-酮膦酸酯的合成. 反应步骤中涉及了锰氧化的膦自由基的生成以及自由基对不饱和键的加成过程. 反应中所需的底物方便易得, 且体系不需要任何酸或碱作为添加剂. 最后进行了克级放大实验, 进一步证明了这一策略在高效合成β-酮膦酸酯中的价值.
霍炳豪, 郭聪慧, 徐占辉. Mn(acac)3促进烯醇酯与亚磷酸酯的自由基氧化偶联反应合成β-酮膦酸酯[J]. 有机化学, 2023, 43(11): 3989-3996.
Binghao Huo, Conghui Guo, Zhanhui Xu. Mn(acac)3 Promoted Radical Oxidative Coupling Reaction of Enol Esters with Phosphites to Synthesize β-Ketophosphonates[J]. Chinese Journal of Organic Chemistry, 2023, 43(11): 3989-3996.
| Entry | Oxidant (equiv.) | Additive (equiv.) | Solvent | Temp./℃ | Time/h | Yieldb/% |
|---|---|---|---|---|---|---|
| 1 | Mn(acac)3 (1.5) | CH3CN | 80 | 6 | 55 | |
| 2 | Mn(OAc)3 (1.5) | CH3CN | 80 | 6 | Trace | |
| 3 | Mn(acac)2 (1.5) | CH3CN | 80 | 6 | 32 | |
| 4 | K2S2O8 (1.5) | CH3CN | 80 | 6 | NDf | |
| 5 | NH4S2O8 (1.5) | CH3CN | 80 | 6 | NDf | |
| 6 | DTBP (1.5) | CH3CN | 80 | 6 | NDf | |
| 7 | TBHP (1.5) | CH3CN | 80 | 6 | NDf | |
| 8 | H2O2 (1.5) | CH3CN | 80 | 6 | Trace | |
| 9 | Mn(acac)3 (0.2)+DTBP (1.5) | CH3CN | 80 | 6 | Trace | |
| 10 | Mn(acac)3 (0.2)+K2S2O8 (1.5) | CH3CN | 80 | 6 | NDf | |
| 11 | Mn(acac)3 (1.5) | 1,4-Dioxane | 80 | 6 | 46 | |
| 12 | Mn(acac)3 (1.5) | THF | 80 | 6 | 38 | |
| 13 | Mn(acac)3 (1.5) | DCE | 80 | 6 | 43 | |
| 14 | Mn(acac)3 (1.5) | EA | 80 | 6 | 58 | |
| 15 | Mn(acac)3 (1.5) | DMF | 80 | 6 | 32 | |
| 16 | Mn(acac)3 (1.5) | NMP | 80 | 6 | Trace | |
| 17 | Mn(acac)3 (2.0) | CH3CN | 80 | 6 | 70 | |
| 18 | Mn(acac)3 (3.0) | CH3CN | 80 | 6 | 81 | |
| 19 | Mn(acac)3 (4.0) | CH3CN | 80 | 6 | 72 | |
| 20 | Mn(acac)3 (3.0) | CH3CN | 80 | 6 | 74c | |
| 21 | Mn(acac)3 (3.0) | CH3CN | 80 | 6 | 85d | |
| 22 | Mn(acac)3 (3.0) | CH3CO2H (2.0) | CH3CN | 80 | 6 | 51 |
| 23 | Mn(acac)3 (3.0) | C4H9CO2H (2.0) | CH3CN | 80 | 6 | 56 |
| 24 | Mn(acac)3 (3.0) | NEt3 (2.0) | CH3CN | 80 | 6 | 61 |
| 25 | Mn(acac)3 (3.0) | DBU (2.0) | CH3CN | 80 | 6 | 48 |
| 26 | Mn(acac)3 (3.0) | — | CH3CN | 60 | 6 | 72 |
| 27 | Mn(acac)3 (3.0) | — | CH3CN | 100 | 6 | 66 |
| 28 | Mn(acac)3 (3.0) | — | CH3CN | 80 | 4 | 69 |
| 29 | Mn(acac)3 (3.0) | — | CH3CN | 80 | 8 | 79 |
| 30e | Mn(acac)3 (3.0) | — | CH3CN | 80 | 6 | 82e |
| Entry | Oxidant (equiv.) | Additive (equiv.) | Solvent | Temp./℃ | Time/h | Yieldb/% |
|---|---|---|---|---|---|---|
| 1 | Mn(acac)3 (1.5) | CH3CN | 80 | 6 | 55 | |
| 2 | Mn(OAc)3 (1.5) | CH3CN | 80 | 6 | Trace | |
| 3 | Mn(acac)2 (1.5) | CH3CN | 80 | 6 | 32 | |
| 4 | K2S2O8 (1.5) | CH3CN | 80 | 6 | NDf | |
| 5 | NH4S2O8 (1.5) | CH3CN | 80 | 6 | NDf | |
| 6 | DTBP (1.5) | CH3CN | 80 | 6 | NDf | |
| 7 | TBHP (1.5) | CH3CN | 80 | 6 | NDf | |
| 8 | H2O2 (1.5) | CH3CN | 80 | 6 | Trace | |
| 9 | Mn(acac)3 (0.2)+DTBP (1.5) | CH3CN | 80 | 6 | Trace | |
| 10 | Mn(acac)3 (0.2)+K2S2O8 (1.5) | CH3CN | 80 | 6 | NDf | |
| 11 | Mn(acac)3 (1.5) | 1,4-Dioxane | 80 | 6 | 46 | |
| 12 | Mn(acac)3 (1.5) | THF | 80 | 6 | 38 | |
| 13 | Mn(acac)3 (1.5) | DCE | 80 | 6 | 43 | |
| 14 | Mn(acac)3 (1.5) | EA | 80 | 6 | 58 | |
| 15 | Mn(acac)3 (1.5) | DMF | 80 | 6 | 32 | |
| 16 | Mn(acac)3 (1.5) | NMP | 80 | 6 | Trace | |
| 17 | Mn(acac)3 (2.0) | CH3CN | 80 | 6 | 70 | |
| 18 | Mn(acac)3 (3.0) | CH3CN | 80 | 6 | 81 | |
| 19 | Mn(acac)3 (4.0) | CH3CN | 80 | 6 | 72 | |
| 20 | Mn(acac)3 (3.0) | CH3CN | 80 | 6 | 74c | |
| 21 | Mn(acac)3 (3.0) | CH3CN | 80 | 6 | 85d | |
| 22 | Mn(acac)3 (3.0) | CH3CO2H (2.0) | CH3CN | 80 | 6 | 51 |
| 23 | Mn(acac)3 (3.0) | C4H9CO2H (2.0) | CH3CN | 80 | 6 | 56 |
| 24 | Mn(acac)3 (3.0) | NEt3 (2.0) | CH3CN | 80 | 6 | 61 |
| 25 | Mn(acac)3 (3.0) | DBU (2.0) | CH3CN | 80 | 6 | 48 |
| 26 | Mn(acac)3 (3.0) | — | CH3CN | 60 | 6 | 72 |
| 27 | Mn(acac)3 (3.0) | — | CH3CN | 100 | 6 | 66 |
| 28 | Mn(acac)3 (3.0) | — | CH3CN | 80 | 4 | 69 |
| 29 | Mn(acac)3 (3.0) | — | CH3CN | 80 | 8 | 79 |
| 30e | Mn(acac)3 (3.0) | — | CH3CN | 80 | 6 | 82e |
| [1] |
(a) Palacios, F.; Alonso, C.; de los Santos, J. M. Chem. Rev. 2005, 105, 899.
doi: 10.1021/cr040672y |
|
(b) Shibata, M.; Ikeda, M.; Motoyama, K.; Miyake, Y.; Nishibayashi, Y. Chem. Commun. 2012, 48, 9528.
doi: 10.1039/c2cc35262a |
|
|
(c) Tao, X.; Li, W.; Li, X.; Xie, X.; Zhang, Z. Org. Lett. 2013, 15, 72.
doi: 10.1021/ol303105d |
|
|
(d) Liu, K.-J.; Ou, J.-H.; Ou, L.-J.; Liu, H.-W.; Tang, X.-D.; Li, L.-B.; Hu, B.-N. Chin. J. Org. Chem. 2015, 35, 1889. (in Chinese)
|
|
|
(刘开建, 欧金花, 欧丽娟, 刘宏伟, 唐新德, 李来丙, 胡波年, 有机化学, 2015, 35, 1889.)
doi: 10.6023/cjoc201502015 |
|
| [2] |
(a) Perumal, S. K.; Adediran, S. A.; Pratt, R. F. Bioorg. Med. Chem. 2008, 16, 6987.
doi: 10.1016/j.bmc.2008.05.045 |
|
(b) Janicki, I.; Kiełbasiński, P.; Szeląg, J.; Glebski, A.; Szczesna- Antczak, M. Bioorg. Chem. 2020, 96, 103548.
doi: 10.1016/j.bioorg.2019.103548 |
|
| [3] |
(a) Sampson, P.; Hammond, G. B.; Wiemer, D. F. J. Org. Chem. 1986, 51, 4342.
doi: 10.1021/jo00373a003 |
|
(b) Corbel, B.; Lhostis-Kervella, I.; Haelters, J. P. Synth. Commun. 2000, 30, 609.
doi: 10.1080/00397910008087362 |
|
| [4] |
(a) Remy, A.; Yannick, L. Tetrahedron Lett. 1997, 38, 233.
doi: 10.1016/S0040-4039(96)02307-6 pmid: 32756671 |
|
(b) Yuan, Y.; Yang, J.; Lei, A. Chem. Soc. Rev. 2021, 50, 10058.
doi: 10.1039/D1CS00150G pmid: 32756671 |
|
|
(c) Huang, H.-M.; Bellotti, P.; Glorius, F. Chem. Soc. Rev. 2020, 49, 6186.
doi: 10.1039/d0cs00262c pmid: 32756671 |
|
| [5] |
Ke, J.; Tang, Y.; Yi, H.; Li, Y.-L.; Cheng, Y.-D.; Liu, C.; Lei, A. Angew. Chem., Int. Ed. 2015, 54, 6604.
doi: 10.1002/anie.v54.22 |
| [6] |
(a) Zhu, Y.-F.; Wei, Y.-Y. Chem. Sci. 2014, 5, 2379.
doi: 10.1039/c4sc00093e pmid: 27934364 |
|
(b) Zhang, W.; Wang, N.-X.; Bai, C.-B.; Wang, Y.-J.; Lan, X.-W.; Xing, Y.-L.; Li, Y.-H.; Wen, J.-L. Sci. Rep. 2015, 5, 15250.
doi: 10.1038/srep15250 pmid: 27934364 |
|
|
(c) Lan, X.-W.; Wang, N.-X.; Bai, C.-B.; Lan, C.-L.; Zhang, T.; Chen, S.-L.; Xing, Y.-L. Org. Lett. 2016, 18, 5986.
pmid: 27934364 |
|
|
(d) Zhu, K.-L.; Dunne, J.; Shaver, M. P.; Thomas, S. P. ACS Catal. 2017, 7, 2353.
doi: 10.1021/acscatal.6b03287 pmid: 27934364 |
|
| [7] |
(a) Goliszewska, K.; Rybicka-Jasińska, K.; Szurmak, J.; Gryko, D. J. Org. Chem. 2019, 84, 15834.
doi: 10.1021/acs.joc.9b02073 pmid: 31594308 |
|
(b) Shang, W.-B.; Peng, F.-Y.; Feng, Q.-L. Org. Chem. Front. 2022, 9, 2680.
doi: 10.1039/D2QO00198E pmid: 31594308 |
|
| [8] |
(a) Tang, Y.-C.; Fan, Y.-Y.; Gao, H.-J.; Li, X.-Q.; Xu, X.-S. Tetrahedron Lett. 2015, 56, 5616.
doi: 10.1016/j.tetlet.2015.08.055 pmid: 31309831 |
|
(b) Liang, X.; Xiong, M.-T.; Zhu, H.-P.; Shen, K.-X.; Pan, Y. J. Org. Chem. 2019, 84, 11210.
doi: 10.1021/acs.joc.9b01400 pmid: 31309831 |
|
|
(c) Bu, M.-J.; Cai, C.; Gallou, F.; Lipshutz, B. H. Green Chem. 2018, 20, 1233.
doi: 10.1039/C7GC03866F pmid: 31309831 |
|
| [9] |
(a) Liwosz, T. W.; Chemler, S. R. Chem.-Eur. J. 2013, 19, 12771.
doi: 10.1002/chem.v19.38 |
|
(b) Pan, X.-Q.; Zou, J.-P.; Zhang, G.-L.; Zhang, W. Chem. Commun. 2010, 46, 1721.
doi: 10.1039/b925951a |
|
| [10] |
(a) Wei, W.; Ji, J.-X. Angew. Chem., Int. Ed. 2011, 50, 9097.
doi: 10.1002/anie.v50.39 |
|
(b) Zhou, Y.; Zhou, M.; Chen, M.; Su, J.; Du, J.; Song, Q. RSC Adv. 2015, 5, 103977.
doi: 10.1039/C5RA23950H |
|
|
(c) Zhang, P.; Zhang, L.; Gao, Y.; Xu, J.; Fang, H.; Tang, G.; Zhao, Y. Chem. Commun. 2015, 51, 7839.
doi: 10.1039/C5CC01904D |
|
| [11] |
(a) Zhou, Y.; Rao, C.; Mai, S.; Song, Q. J. Org. Chem. 2016, 81, 2027.
doi: 10.1021/acs.joc.5b02887 pmid: 29136376 |
|
(b) Zhou, P.; Hu, B.; Li, L.; Rao, K.; Yang, J.; Yu, F. J. Org. Chem. 2017, 82, 13268.
doi: 10.1021/acs.joc.7b02391 pmid: 29136376 |
|
| [12] |
(a) Fu, M.-C.; Shang, R.; Zhao, B.; Wang, B.; Fu, Y. Science 2019, 363, 1429.
doi: 10.1126/science.aav3200 |
|
(b) Kong, W.; Yu, C.; An, H.; Song, Q. Org. Lett. 2018, 20, 349.
doi: 10.1021/acs.orglett.7b03587 |
|
|
(c) Zhao, B.; Shang, R.; Wang, G.-Z.; Wang, S.; Chen, H.; Fu, Y. ACS. Catal. 2020, 10, 1334.
doi: 10.1021/acscatal.9b04699 |
|
|
(d) Feng, Z.; Zhu, B.; Dong, B.; Cheng, L.; Li, Y.; Wang, Z.; Wu, J. Org. Lett. 2021, 23, 508.
doi: 10.1021/acs.orglett.0c04021 |
|
| [13] |
Zhou, P.; Hu, B.; Li, L.; Rao, K.; Yang, J.; Yu, F. J. Org. Chem. 2017, 82, 13268.
doi: 10.1021/acs.joc.7b02391 pmid: 29136376 |
| [14] |
Liu, Y.; Li, S.-J.; Chen, X.-L.; Fan, L.-L.; Li, X.-Y.; Zhu, S.-S.; Qu, L.-B.; Yu, B. Adv. Synth. Catal. 2020, 362, 688.
doi: 10.1002/adsc.v362.3 |
| [15] |
(a) Li, W.-P.; Zhu, Y.-C.; Zhou, Y.-J.; Yang, H.-W.; Zhu, C.-J. Tetrahedron 2019, 75, 1647.
doi: 10.1016/j.tet.2018.12.023 |
|
(b) Garg, P.; Singh, A. Asian J. Org. Chem. 2019, 8, 849.
doi: 10.1002/ajoc.v8.6 |
|
|
(c) Taniguchi, R.; Noto, N.; Tanaka, S.; Takahashi, K.; Oyama, R.; Abe, M.; Koike, T.; Akita, M. Chem. Commun. 2021, 57, 2609.
doi: 10.1039/D0CC08060H |
|
| [16] |
(a) Gärtner, D.; Stein, A. L.; Grupe, S.; Arp, J.; von Wangelin, A. J. Angew. Chem., Int. Ed. 2015, 54, 10545.
doi: 10.1002/anie.v54.36 |
|
(b) Yu, J.-Y.; Shimizu, R.; Kuwano, R. Angew. Chem., Int. Ed. 2010, 49, 6396.
doi: 10.1002/anie.v49:36 |
|
|
(c) Song, C.-X.; Cai, G.-X.; Farrell, T. R.; Jiang, Z.-P.; Li, H.; Gan, L.-B.; Shi, Z.-J. Chem. Commun. 2009, 45, 6002.
|
|
|
(d) Lu, Y.; Li, Y.; Zhang, R.; Jin, K.; Duan, C. J. Fluorine Chem. 2014, 161, 128.
doi: 10.1016/j.jfluchem.2014.01.020 |
|
|
(e) Geibel, I.; Dierks, A.; Müller, T., Christoffers, J. Chem. Eur. J. 2017, 23, 7245.
doi: 10.1002/chem.v23.30 |
|
| [17] |
(a) Chen, X.; Chen, X.-L.; Li, X.; Qu, C.; Qu, L.-B.; Bi, W.-Z.; Sun, K.; Zhao, Y.-F. Tetrahedron 2017, 73. 2459.
|
|
(b) Li, L.-L.; Huang, W.-B.; Chen, L.-J.; Dong, J.-X.; Ma, X.-B.; Peng, Y.-G. Angew. Chem., Int. Ed. 2017, 56, 10539.
doi: 10.1002/anie.v56.35 |
|
|
(c) Yamamoto, D.; Ansai, H.; Hoshino, J.; Makino, K. Chem. Pharm. Bull. 2018, 66, 873.
doi: 10.1248/cpb.c18-00381 |
|
|
(d) Yi, N.-N.; Wang, R.; Zou, H.; He, W.-B.; Fu, W.-Q.; He, W.-M. J. Org. Chem. 2015, 80, 5023.
doi: 10.1021/acs.joc.5b00408 |
|
|
(e) Zhang, G.-Y.; Li, C.-K.; Li, D.-P.; Zeng, R.-S.; Shoberu, A.; Zou, J.-P. Tetrahedron 2016, 72, 2972.
doi: 10.1016/j.tet.2016.04.013 |
| [1] | 童红恩, 郭宏宇, 周荣. 可见光促进惰性碳-氢键对羰基的加成反应进展[J]. 有机化学, 2024, 44(1): 54-69. |
| [2] | 张建涛, 张聪, 莫诺琳, 罗佳婷, 陈莲芬, 刘卫兵. 氯仿参与的烯烃自由基加成反应的研究进展[J]. 有机化学, 2023, 43(9): 3098-3106. |
| [3] | 马佳敏, 李姣兄, 孟千森, 曾祥华. 炔烃的自由基砜基化反应研究进展[J]. 有机化学, 2023, 43(6): 2040-2052. |
| [4] | 魏文婷, 李壮壮, 李婉迪, 李嘉琪, 石先莹. 纯水及空气中芳香羧酸和丙烯酸酯氧化偶联构筑苯酞的绿色方法[J]. 有机化学, 2023, 43(3): 1177-1186. |
| [5] | 沈梦涵, 李来强, 周泉, 王洁慧, 王磊. 可见光诱导下喹喔啉酮与吡咯衍生物的氧化偶联[J]. 有机化学, 2023, 43(2): 697-704. |
| [6] | 赵健铭, 朱佳顺, 沈佳斌, 张怡岚, 李万梅. 光催化氧化交叉偶联制备对称/不对称硫代磺酸酯[J]. 有机化学, 2022, 42(9): 2940-2946. |
| [7] | 尹艳丽, 赵筱薇, 江智勇. 可见光不对称催化合成手性氮杂芳烃衍生物[J]. 有机化学, 2022, 42(6): 1609-1625. |
| [8] | 孙鑫, 屈超凡, 马超蕊, 赵筱薇, 柴国璧, 江智勇. 光氧化还原催化串联自由基加成反应构建1,4-二酮官能团化喹喔啉-2(1H)-酮衍生物[J]. 有机化学, 2022, 42(5): 1396-1406. |
| [9] | 高启升, 荆祺, 陈阳, 孙京, 周明东. N-苯基丙烯酰胺与草氨酸衍生物的脱羧氨甲酰化反应研究[J]. 有机化学, 2022, 42(1): 257-265. |
| [10] | 刘锐凯, 许峥, 宁志涛, 杜正银. 碘催化乙烯基叠氮与芳基亚磺酸钠反应合成β-磺酰基烯胺[J]. 有机化学, 2022, 42(1): 200-207. |
| [11] | 钱俊峰, 田晓婷, 吴中, 姚杰, 王慧, 周维友. 非均相含镍层状双金属氧化物催化异色满与芳伯胺的高效氧化偶联[J]. 有机化学, 2021, 41(9): 3668-3674. |
| [12] | 陈伟, 刘强. 烯醇类化合物氧化偶联反应研究进展[J]. 有机化学, 2021, 41(9): 3414-3430. |
| [13] | 杜科莹, 张展铭, 盛卫坚. 铜催化2-羟基芳基烯胺酮经三氟甲基自由基加成串联环化反应合成3-三氟甲基色酮[J]. 有机化学, 2021, 41(8): 3242-3248. |
| [14] | 张晓平, 金桂勇, 陈芝飞, 王清福, 赵森森, 武志勇, 万帅, 席高磊, 赵旭. 吡嗪-噻唑联芳类化合物的合成及抗氧化性能研究[J]. 有机化学, 2021, 41(6): 2445-2453. |
| [15] | 孙建婷, 陈玲艳, 魏邦国. 二碘化钐促进的2-哌啶酮与α,β-不饱和酸酯的加成-开环反应研究[J]. 有机化学, 2021, 41(11): 4320-4326. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||