有机化学 ›› 2023, Vol. 43 ›› Issue (2): 697-704.DOI: 10.6023/cjoc202207031 上一篇 下一篇
研究论文
沈梦涵a, 李来强a, 周泉a,*( ), 王洁慧a, 王磊a,b,*(
), 王洁慧a, 王磊a,b,*( )
)
收稿日期:2022-07-25
修回日期:2022-09-15
发布日期:2022-10-24
基金资助:
Menghan Shena, Laiqiang Lia, Quan Zhoua( ), Jiehui Wanga, Lei Wanga,b(
), Jiehui Wanga, Lei Wanga,b( )
)
Received:2022-07-25
Revised:2022-09-15
Published:2022-10-24
Contact:
*E-mail: Supported by:文章分享
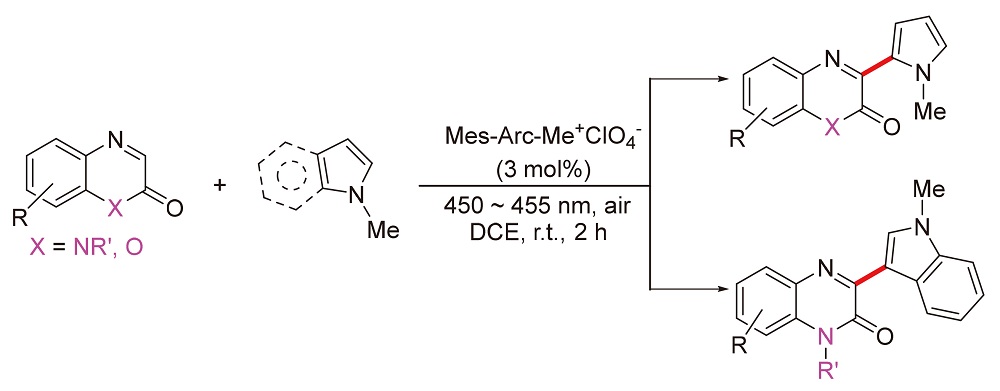
发展了一种区域选择性的喹喔啉酮3-位氧化偶联富电子吡咯衍生物的方法. 以$\text{Mes-Acr-M}{{\text{e}}^{\text{+}}}\text{ClO}_{\text{4}}^{-}$(3 mol%)为光敏剂, 空气氧为氧化剂, 高收率得到喹喔酮与吡咯衍生物的氧化偶联产物. 该方法底物的官能团适用范围广, 反应高效绿色, 可快速构建基于喹喔啉酮-吡咯衍生物的药物分子库. 相同测试条件下的Stern-Volmer荧光淬灭实验表明, 缺电子芳烃1-甲基喹喔啉酮对光敏剂的淬灭速率常数Kq=1.2×109 L•mol-1•s-1, 而富电子芳烃1-甲基吡咯/1-甲基吲哚的淬灭常数约为1.06~1.07×1010 L•mol-1•s-1, 后者的荧光淬灭速率几乎是前者的10倍.
沈梦涵, 李来强, 周泉, 王洁慧, 王磊. 可见光诱导下喹喔啉酮与吡咯衍生物的氧化偶联[J]. 有机化学, 2023, 43(2): 697-704.
Menghan Shen, Laiqiang Li, Quan Zhou, Jiehui Wang, Lei Wang. Visible-Light-Induced Regio-selective Oxidative Coupling of Quinoxalinones with Pyrrole Derivatives[J]. Chinese Journal of Organic Chemistry, 2023, 43(2): 697-704.
| Entry | Photocatalyst | Light source/nm | Solvent | Yieldb/% |
|---|---|---|---|---|
| 1 | TPT | 450~455 | DCE | 48 |
| 2 | Mes-Acr-Me+ClO4‒ | 450~455 | DCE | 75 |
| 3 | Ru(bpy)3Cl2 | 420~425 | DCE | 0 |
| 4 | 4CzIPN | 420~425 | DCE | 0 |
| 5 | Eosin Y | 530~535 | DCE | 58 |
| 6 | $\text{Mes-Acr-M}{{\text{e}}^{\text{+}}}\text{ClO}_{\text{4}}^{-}$ | 450~455 | THF | 12 |
| 7 | $\text{Mes-Acr-M}{{\text{e}}^{\text{+}}}\text{ClO}_{\text{4}}^{-}$ | 450~455 | Acetone | 15 |
| 8 | $\text{Mes-Acr-M}{{\text{e}}^{\text{+}}}\text{ClO}_{\text{4}}^{-}$ | 450~455 | MeCN | 11 |
| 9 | $\text{Mes-Acr-M}{{\text{e}}^{\text{+}}}\text{ClO}_{\text{4}}^{-}$ | 450~455 | DMSO | 5 |
| 10 | $\text{Mes-Acr-M}{{\text{e}}^{\text{+}}}\text{ClO}_{\text{4}}^{-}$ | - | DCE | 0c |
| 11 | $\text{Mes-Acr-M}{{\text{e}}^{\text{+}}}\text{ClO}_{\text{4}}^{-}$ | 450~455 | DCE | 0d |
| Entry | Photocatalyst | Light source/nm | Solvent | Yieldb/% |
|---|---|---|---|---|
| 1 | TPT | 450~455 | DCE | 48 |
| 2 | Mes-Acr-Me+ClO4‒ | 450~455 | DCE | 75 |
| 3 | Ru(bpy)3Cl2 | 420~425 | DCE | 0 |
| 4 | 4CzIPN | 420~425 | DCE | 0 |
| 5 | Eosin Y | 530~535 | DCE | 58 |
| 6 | $\text{Mes-Acr-M}{{\text{e}}^{\text{+}}}\text{ClO}_{\text{4}}^{-}$ | 450~455 | THF | 12 |
| 7 | $\text{Mes-Acr-M}{{\text{e}}^{\text{+}}}\text{ClO}_{\text{4}}^{-}$ | 450~455 | Acetone | 15 |
| 8 | $\text{Mes-Acr-M}{{\text{e}}^{\text{+}}}\text{ClO}_{\text{4}}^{-}$ | 450~455 | MeCN | 11 |
| 9 | $\text{Mes-Acr-M}{{\text{e}}^{\text{+}}}\text{ClO}_{\text{4}}^{-}$ | 450~455 | DMSO | 5 |
| 10 | $\text{Mes-Acr-M}{{\text{e}}^{\text{+}}}\text{ClO}_{\text{4}}^{-}$ | - | DCE | 0c |
| 11 | $\text{Mes-Acr-M}{{\text{e}}^{\text{+}}}\text{ClO}_{\text{4}}^{-}$ | 450~455 | DCE | 0d |
| [1] |
(a) Han, W. B.; Lu, Y. H.; Zhang, A. H.; Zhang, G. F.; Mei, Y. N.; Jiang, N.; Lei, X.; Song, Y. C.; Ng, S. W.; Tan, R. X. Org. Lett. 2014, 16, 5366.
doi: 10.1021/ol502572g |
|
(b) Michael, J. P. Nat. Pro. Rep. 2007, 24, 191.
|
|
|
(c) Zhang, J.; Morris-Natschke, S. L.; Ma, D.; Shang, X.-F.; Yang, C.-J.; Liu, Y.-Q.; Lee, K.-H. Med. Res. Rev. 2021, 41, 928.
doi: 10.1002/med.21747 |
|
| [2] |
Liang, F.; Eda, K.; Okazoe, T.; Wada, A.; Mori, N.; Konishi, K.; Tsuda, A. J. Org. Chem. 2021, 86, 6504.
doi: 10.1021/acs.joc.1c00334 |
| [3] |
Tucker, J. W.; Narayanam, J. M. R.; Krabbe, S. W.; Stephenson, C. R. J. Org. Lett. 2010, 12, 368.
doi: 10.1021/ol902703k |
| [4] |
Ghosh, I.; Ghosh, T.; Bardagi, J. I.; König, B. Science 2014, 346, 725.
doi: 10.1126/science.1258232 |
| [5] |
(a) Kim, H.; Kim, H.; Lambert, T. H.; Lin, S. J. Am. Chem. Soc. 2020, 142, 2087.
doi: 10.1021/jacs.9b10678 pmid: 29131437 |
|
(b) Cowper, N. G. W. C.; Chernowsky, P.; Williams, O. P.; Wickens, Z. K. J. Am. Chem. Soc. 2020, 142, 2093.
doi: 10.1021/jacs.9b12328 pmid: 29131437 |
|
|
(c) Neumeier, M.; Sampedro, D.; Májek, M.; de la Peña O'Shea, V. A.; Jacobi von Wangelin, A.; Pérez-Ruiz, R. Chem.-Eur. J. 2018, 24, 105.
doi: 10.1002/chem.201705326 pmid: 29131437 |
|
|
(d) Caby, S.; Bouchet, L. M.; Argüello, J. E.; Rossi, R. A.; Bardagi, J. I. ChemCatChem 2021, 13, 3001.
doi: 10.1002/cctc.202100359 pmid: 29131437 |
|
|
(e) Ghosh, I.; Shaikh, R. S.; König, B. Angew. Chem., Int. Ed. 2017, 56, 8544.
doi: 10.1002/anie.201703004 pmid: 29131437 |
|
|
(f) Ghosh, I.; König, B. Angew. Chem., Int. Ed. 2016, 55, 7676.
doi: 10.1002/anie.201602349 pmid: 29131437 |
|
|
(g) Chernowsky, C. P.; Chmiel, A. F.; Wickens, Z. K. Angew. Chem., Int. Ed. 2021, 60, 21418.
doi: 10.1002/anie.202107169 pmid: 29131437 |
|
|
(h) Ding, Y.-X.; Zhang, R.-Q.; Ma, R.-C.; Ma, Y.-M. Adv. Synth. Catal. 2022, 364, 355.
doi: 10.1002/adsc.202100991 pmid: 29131437 |
|
| [6] |
Li, Z.-J.; Li, S.; Hofman, E.; Hunter Davis, A.; Leem, G.; Zheng, W. Green Chem. 2020, 22, 1911.
doi: 10.1039/C9GC04248B |
| [7] |
(a) Carta, A.; Piras, S.; Loriga, G.; Paglietti, G. Mini-Rev. Med. Chem. 2006, 6, 1179.
pmid: 17100630 |
|
(b) Liu, R.; Huang, Z.; Murray, M. G.; Guo, X.; Liu, G. J. Med. Chem. 2011, 54, 5747.
doi: 10.1021/jm200394x pmid: 17100630 |
|
| [8] |
Carrër, A.; Brion, J.-D.; Messaoudi, S.; Alami, M. Org. Lett. 2013, 15, 5606.
doi: 10.1021/ol4028946 |
| [9] |
Ramesh, B.; Reddy, C. R.; Kumar, G. R.; Reddy, B. V. S. Tetrahedron Lett. 2018, 59, 628.
doi: 10.1016/j.tetlet.2017.12.085 |
| [10] |
(a) Hong, Y.-Y.; Peng, Z.; Ma, H.; Zhu, Q.; Xu, X.-Q.; Yang, L.-H.; Xie, L.-Y. Tetrahedron Lett. 2022, 89, 153595.
doi: 10.1016/j.tetlet.2021.153595 |
|
(b) Kishor, G.; Ramesh, V.; Rao, V. R.; Pabbaraja, S.; Adiyala, P. R. RSC Adv. 2022, 12, 12235.
doi: 10.1039/D2RA00753C |
|
|
(c) Ni, H.; Li, Y.; Deng, J.; Shi, X.; Pan, Q. New J. Chem. 2021, 45, 22432.
doi: 10.1039/D1NJ04805H |
|
|
(d) Sun, K.; Shi, A.; Liu, Y.; Chen, X.; Xiang, P.; Wang, X.; Qu, L.; Yu, B. Chem. Sci. 2022, 13, 5659.
doi: 10.1039/D2SC01241C |
|
|
(e) Xie, L.-Y.; Bai, Y.-S.; Xu, X.-Q.; Peng, X.; Tang, H.-S.; Huang, Y.; Lin, Y.-W.; Cao, Z.; He, W.-M. Green Chem. 2020, 22, 1720.
doi: 10.1039/C9GC03899J |
|
| [11] |
(a) Yuan, J.; Liu, S.; Qu, L. Adv. Syn. Catal. 2017, 359, 4197.
doi: 10.1002/adsc.201701058 pmid: 32392413 |
|
(b) Yuan, J.-W.; Fu, J.-H.; Liu, S.-N.; Xiao, Y.-M.; Mao, P.; Qu, L.-B. Org. Biomol. Chem. 2018, 16, 3203.
doi: 10.1039/C8OB00206A pmid: 32392413 |
|
|
(c) Xie, L.-Y.; Peng, S.; Fan, T.-G.; Liu, Y.-F.; Sun, M.; Jiang, L.-L.; Wang, X.-X.; Cao, Z.; He, W.-M. Sci. China: Chem. 2019, 62, 460.
pmid: 32392413 |
|
|
(d) Meng, N.; Wang, L.; Liu, Q.; Li, Q.; Lv, Y.; Yue, H.; Wang, X.; Wei, W. J. Org. Chem. 2020, 85, 6888.
doi: 10.1021/acs.joc.9b03505 pmid: 32392413 |
|
| [12] |
(a) Niu, K.; Ding, L.; Zhou, P.; Hao, Y.; Liu, Y.; Song, H.; Wang, Q. Green Chem. 2021, 23, 3246.
doi: 10.1039/D1GC00861G |
|
(b) Niu, K.; Song, L.; Hao, Y.; Liu, Y.; Wang, Q. Chem. Commun. 2020, 56, 11673.
doi: 10.1039/D0CC05391K |
|
|
(c) Gao, Y.; Wu, Z.; Yu, L.; Wang, Y.; Pan, Y. Angew. Chem., Int. Ed. 2020, 59, 10859.
doi: 10.1002/anie.202001571 |
|
|
(d) Lin, D.-Z.; Huang, J.-M. Org. Lett. 2019, 21, 5862.
doi: 10.1021/acs.orglett.9b01971 |
|
|
(e) Lei, N.; Shen, Y.; Li, Y.; Tao, P.; Yang, L.; Su, Z.; Zheng, K. Org. Lett. 2020, 22, 9184.
doi: 10.1021/acs.orglett.0c03158 |
|
| [13] |
(a) Clinton, C. D.; Prasad, C. D.; Thombal, R. S.; Lee, Y. R. Adv. Syn. Catal. 2021, 363, 776.
doi: 10.1002/adsc.202001182 |
|
(b) Ni, H.; Li, Y.; Shi, X.; Pang, Y.; Jin, C.; Zhao, F. Tetrahedron Lett. 2021, 68, 152915.
doi: 10.1016/j.tetlet.2021.152915 |
|
|
(c) Xu, J.; Yang, H.; He, L.; Huang, L.; Shen, J.; Li, W.; Zhang, P. Org. Lett. 2021, 23, 195.
doi: 10.1021/acs.orglett.0c03918 |
|
| [14] |
(a) Tan, Y.; Liu, B.; Han, Y.-P.; Zhang, Y.; Zhang, H.-Y.; Zhao, J. Chem.-Asian J. 2020, 15, 3365.
doi: 10.1002/asia.202000916 pmid: 30372088 |
|
(b) Wei, W.; Wang, L.; Bao, P.; Shao, Y.; Yue, H.; Yang, D.; Yang, X.; Zhao, X.; Wang, H. Org. Lett. 2018, 20, 7125.
doi: 10.1021/acs.orglett.8b03079 pmid: 30372088 |
|
|
(c) Yuan, J.-W.; Zhu, J.-L.; Li, B.; Yang, L.-Y.; Mao, P.; Zhang, S.-R.; Li, Y.-C.; Qu, L.-B. Org. Biomol. Chem. 2019, 17, 10178.
doi: 10.1039/C9OB02157D pmid: 30372088 |
|
| [15] |
(a) Jiang, X.; Yang, L.; Ye, Z.; Du, X.; Fang, L.; Zhu, Y.; Chen, K.; Li, J.; Yu, C. Eur. J. Org. Chem. 2020, 2020, 1687.
doi: 10.1002/ejoc.201901928 |
|
(b) Xu, J.; Yang, H.; Cai, H.; Bao, H.; Li, W.; Zhang, P. Org. Lett. 2019, 21, 4698.
doi: 10.1021/acs.orglett.9b01578 |
|
|
(c) Zhao, L.; Wang, L.; Gao, Y.; Wang, Z.; Li, P. Adv. Syn. Catal. 2019, 361, 5363.
doi: 10.1002/adsc.201900732 |
|
|
(d) Jin, J.; Tong, J.; Yu, W.; Qiao, J.; Shen, C. Catal. Commun. 2020, 141, 106008.
doi: 10.1016/j.catcom.2020.106008 |
|
|
(e) Wang, L.-F.; Li, S.; Xiao, X.-Q.; Xu, W.-M.; Zhang, P.-F.; Ma, Y.-M. Adv. Synth. Catal. 2022, 364, 855.
doi: 10.1002/adsc.202101324 |
|
| [16] |
(a) Teng, Q.-H.; Yao, Y.; Wei, W.-X.; Tang, H.-T.; Li, J.-R.; Pan, Y.-M. Green Chem. 2019, 21, 6241.
doi: 10.1039/C9GC03045J |
|
(b) Zhou, J.; Li, Z.; Sun, Z.; Ren, Q.; Zhang, Q.; Li, H.; Li, J. J. Org. Chem. 2020, 85, 4365.
doi: 10.1021/acs.joc.0c00050 |
|
|
(c) Zhou, J.; Zhou, P.; Zhao, T.; Ren, Q.; Li, J. Adv. Syn. Catal. 2019, 361, 5371.
doi: 10.1002/adsc.201901008 |
|
| [17] |
(a) Kim, Y.; Kim, D. Y. Tetrahedron Lett. 2018, 59, 2443.
doi: 10.1016/j.tetlet.2018.05.034 |
|
(b) Li, K.-J.; Jiang, Y.-Y.; Xu, K.; Zeng, C.-C.; Sun, B.-G. Green Chem. 2019, 21, 4412.
doi: 10.1039/C9GC01474H |
|
|
(c) Rawat, D.; Kumar, R.; Subbarayappa, A. Green Chem. 2020, 22, 6170.
doi: 10.1039/D0GC02168G |
|
|
(d) Zhang, J.-Q.; Hu, D.; Song J.; Ren, H. J. Org. Chem. 2021, 86, 4646.
doi: 10.1021/acs.joc.0c03041 |
|
|
(e) Zhang, J.-Q.; Liu, J.; Hu, D.; Song, J.; Zhu, G.; Ren, H. Org. Lett. 2022, 24, 786.
doi: 10.1021/acs.orglett.1c04336 |
|
| [18] |
Dai, C.; Zhan, Y.; Liu, P.; Sun, P. Green Chem. 2021, 23, 314.
doi: 10.1039/D0GC03697H |
| [19] |
Chupakhin, O. N.; Sidorov, E. O.; Postovskii, I. Y. Khim. Geterotsikl. Soedin. 1975, 10, 1433.
|
| [20] |
Noikham, M.; Kittikool, T.; Yotphan, S. Synthesis 2018, 50, 2337.
doi: 10.1055/s-0037-1609445 |
| [21] |
Utepova, I. A.; Trestsova, M. A.; Chupakhin, O. N.; Charushin, V. N.; Rempel, A. A. Green Chem. 2015, 17, 4401.
doi: 10.1039/C5GC00753D |
| [22] |
Romero, N. A.; Nicewicz, D. A. Chem. Rev. 2016, 116, 10075.
doi: 10.1021/acs.chemrev.6b00057 |
| [23] |
Akaba, R.; Ohshima, K.; Kawai, Y.; Obuchi, Y.; Negishi, A.; Sakuragi, H.; Tokumaru, K. Tetrahedron Lett. 1991, 32, 109.
|
| [24] |
Sadki, S.; Schottland, P.; Brodie, N.; Sabouraud, G. Chem. Soc. Rev. 2000, 29, 283.
doi: 10.1039/a807124a |
| [25] |
Benniston, A. C.; Harriman, A.; Li, P.; Rostron, J. P.; Ramesdonk, H. J.; Groeneveld, M. M.; Zhang, H.; Verhoeven, J. W. J. Am. Chem. Soc. 2005, 127, 16054.
pmid: 16287292 |
| [26] |
Speckmeier, E.; Fischer, T. G.; Zeitler, K. J. Am. Chem. Soc. 2018, 140, 15353.
doi: 10.1021/jacs.8b08933 pmid: 30277767 |
| [27] |
(a) Wang, B.; Zou, L.; Wang, L.; Sun, M.; Li, P. Chin. Chem. Lett. 2021, 32, 1229.
doi: 10.1016/j.cclet.2020.08.013 |
|
(b) Wang, Z.; Wang, L.; Wang, Z.; Li, P.; Zhang, Y. Chin. Chem. Lett. 2021, 32, 429.
doi: 10.1016/j.cclet.2020.02.022 |
|
|
(c) Xie, X.; Wang, L.; Zhou, Q.; Ma, Y.; Wang, Z.-M.; Li, P. Chin. Chem. Lett. 2022, 33, 5069.
doi: 10.1016/j.cclet.2022.03.084 |
|
|
(d) Zhou, Q.; Yu, H.-Y.; Zhou, Y.; Wei, J.-R.; Wang, L. Org. Biomol. Chem. 2022, 20, 5575.
doi: 10.1039/D2OB00639A |
| [1] | 付雅彤, 孙超凡, 张丹, 金成国, 陆居有. 巢式-碳硼烷硼氢键官能化反应研究进展[J]. 有机化学, 2024, 44(2): 438-447. |
| [2] | 张剑, 梁万洁, 杨艺, 闫法超, 刘会. 联烯胺化合物的区域选择性双官能团化[J]. 有机化学, 2024, 44(2): 335-348. |
| [3] | 刘继宇, 李圣玉, 陈款, 朱茵, 张元. 三苯胺功能化有序介孔聚合物作为无金属光催化剂用于二硫化物合成[J]. 有机化学, 2024, 44(2): 605-612. |
| [4] | 梅青刚, 李清寒. 可见光促进C(3)(杂)芳硫基吲哚化合物的合成研究进展[J]. 有机化学, 2024, 44(2): 398-408. |
| [5] | 朱彦硕, 王红言, 舒朋华, 张克娜, 王琪琳. 烷氧自由基引发1,5-氢原子转移实现C(sp3)—H键官能团化的研究进展[J]. 有机化学, 2024, 44(1): 1-17. |
| [6] | 赵红琼, 于淼, 宋冬雪, 贾琦, 刘颖杰, 季宇彬, 许颖. 羧酸脱羧羟基化反应研究进展[J]. 有机化学, 2024, 44(1): 70-84. |
| [7] | 金玉坤, 任保轶, 梁福顺. 可见光介导的三氟甲基的选择性C-F键断裂及其在偕二氟类化合物合成中的应用[J]. 有机化学, 2024, 44(1): 85-110. |
| [8] | 童红恩, 郭宏宇, 周荣. 可见光促进惰性碳-氢键对羰基的加成反应进展[J]. 有机化学, 2024, 44(1): 54-69. |
| [9] | 董江湖, 宣良明, 王池, 赵晨熙, 王海峰, 严琼姣, 汪伟, 陈芬儿. 无过渡金属或无光催化剂条件下可见光促进喹喔啉酮C(3)—H官能团化研究进展[J]. 有机化学, 2024, 44(1): 111-136. |
| [10] | 王灵娜, 刘晓庆, 林钢, 金泓颖, 焦民均, 刘雪粉, 罗书平. 光促进双(4-二苯甲酮)苯醚催化C(sp3)—H键活化构建C—S键[J]. 有机化学, 2023, 43(8): 2848-2854. |
| [11] | 赵瑜, 张凯, 白育斌, 张琰图, 史时辉. 无金属条件下可见光催化与溴盐协同促进烯烃的氢硅化反应研究[J]. 有机化学, 2023, 43(8): 2837-2847. |
| [12] | 杨晓娜, 郭宏宇, 周荣. 可见光促进有机硅化合物参与的化学转化[J]. 有机化学, 2023, 43(8): 2720-2742. |
| [13] | 普佳霞, 贾小英, 韩丽荣, 李清寒. 可见光诱导C—N键断裂构建C—C键的研究进展[J]. 有机化学, 2023, 43(8): 2591-2613. |
| [14] | 刘颖杰, 石岗庆, 仇格, 张鑫, 宋冬雪, 陈宁, 于淼, 许颖. 光/电催化醚α-位官能团化研究进展[J]. 有机化学, 2023, 43(8): 2664-2681. |
| [15] | 范威. O2促进下五元环烯胺的C—H亚胺化[J]. 有机化学, 2023, 43(7): 2492-2498. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||