有机化学 ›› 2024, Vol. 44 ›› Issue (10): 3117-3135.DOI: 10.6023/cjoc202404039 上一篇 下一篇
所属专题: 二氧化碳专题合集
综述与进展
侯静a,*( ), 黄燕b,*(
), 黄燕b,*( ), 李浩a, 万远翠a, 邵雨a, 詹乐武a, 王定海a, 李斌栋a
), 李浩a, 万远翠a, 邵雨a, 詹乐武a, 王定海a, 李斌栋a
收稿日期:2024-04-24
修回日期:2024-07-16
发布日期:2024-08-16
基金资助:
Jing Houa,*( ), Yan Huangb,*(
), Yan Huangb,*( ), Hao Lia, Yuancui Wana, Yu Shaoa, Lewu Zhana, Dinghai Wanga, Bindong Lia
), Hao Lia, Yuancui Wana, Yu Shaoa, Lewu Zhana, Dinghai Wanga, Bindong Lia
Received:2024-04-24
Revised:2024-07-16
Published:2024-08-16
Contact:
*E-mail: Supported by:文章分享

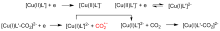
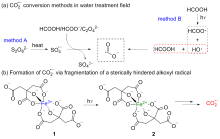
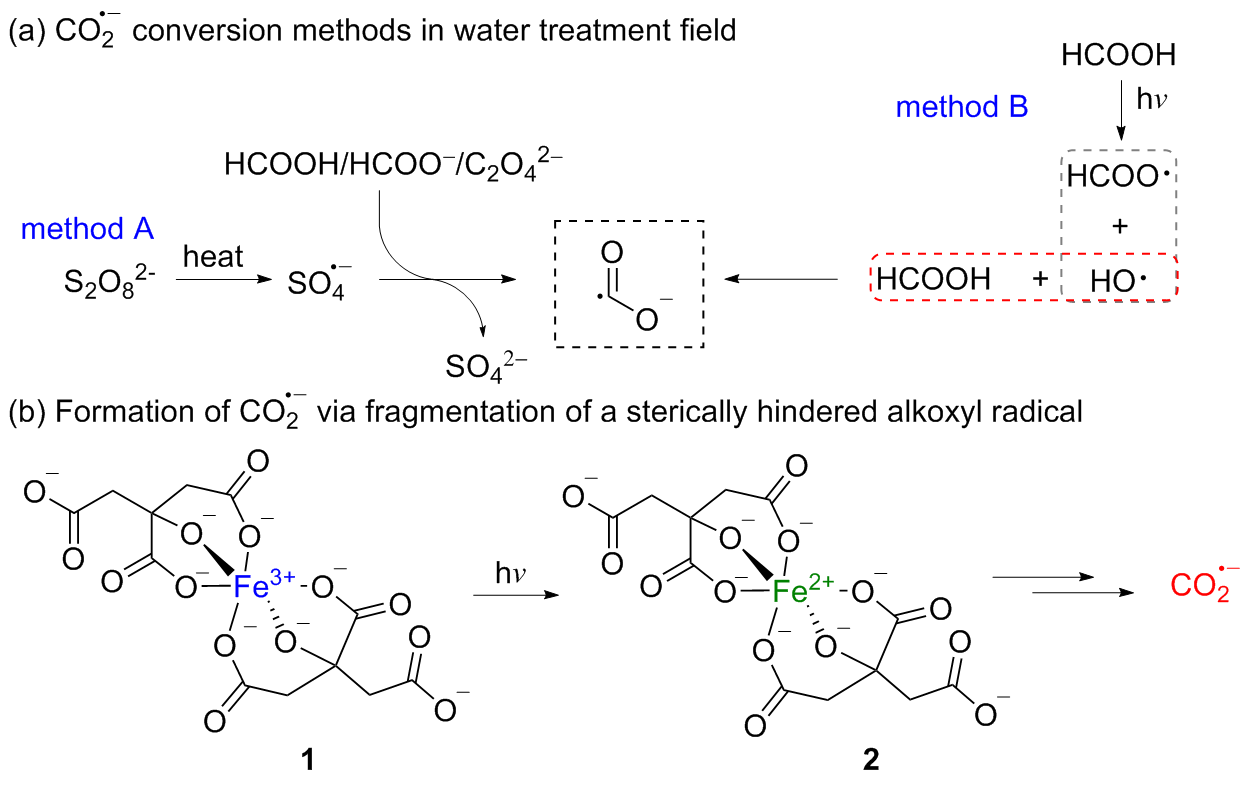
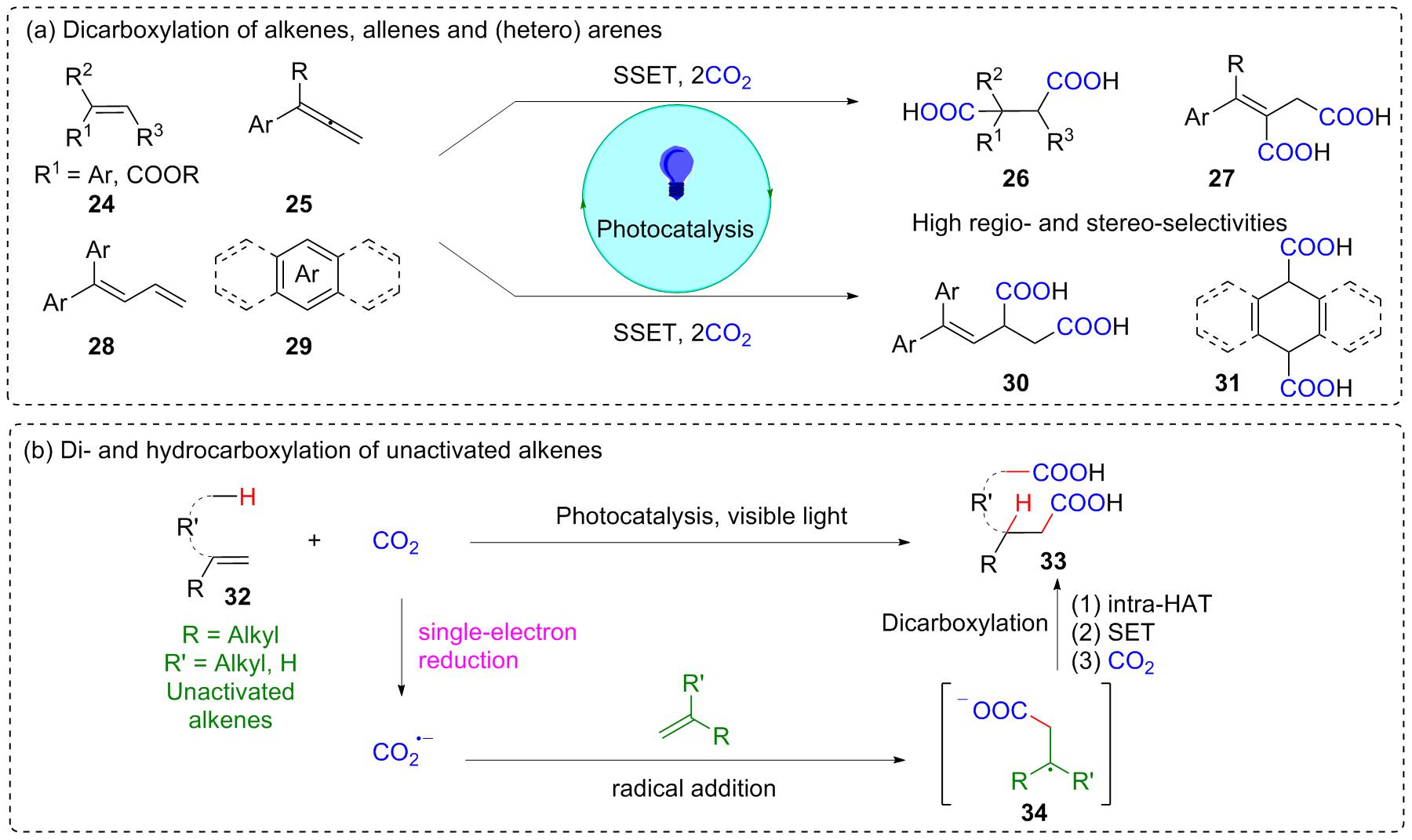
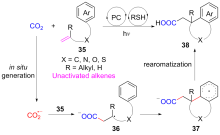
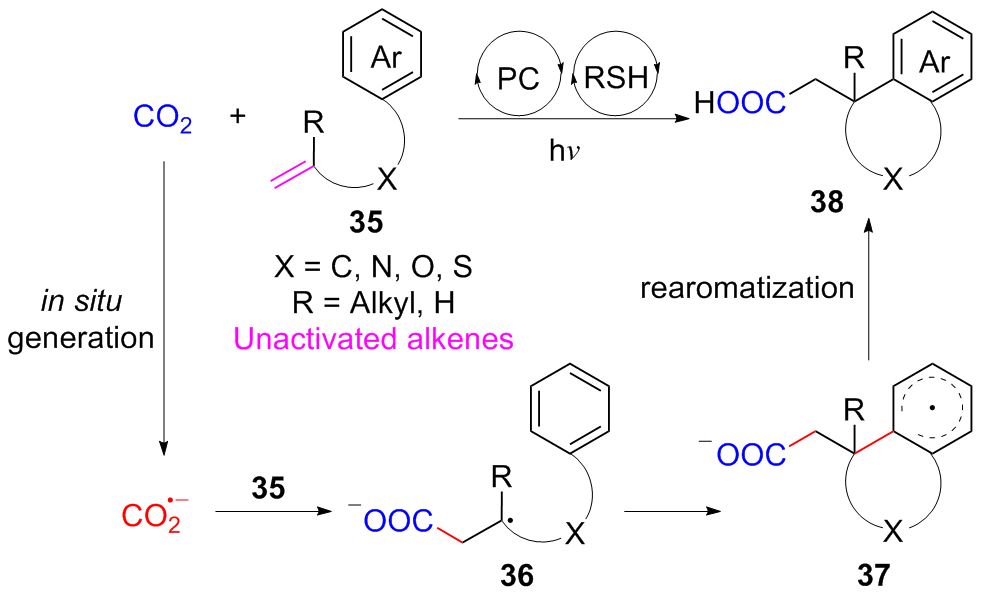
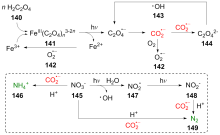
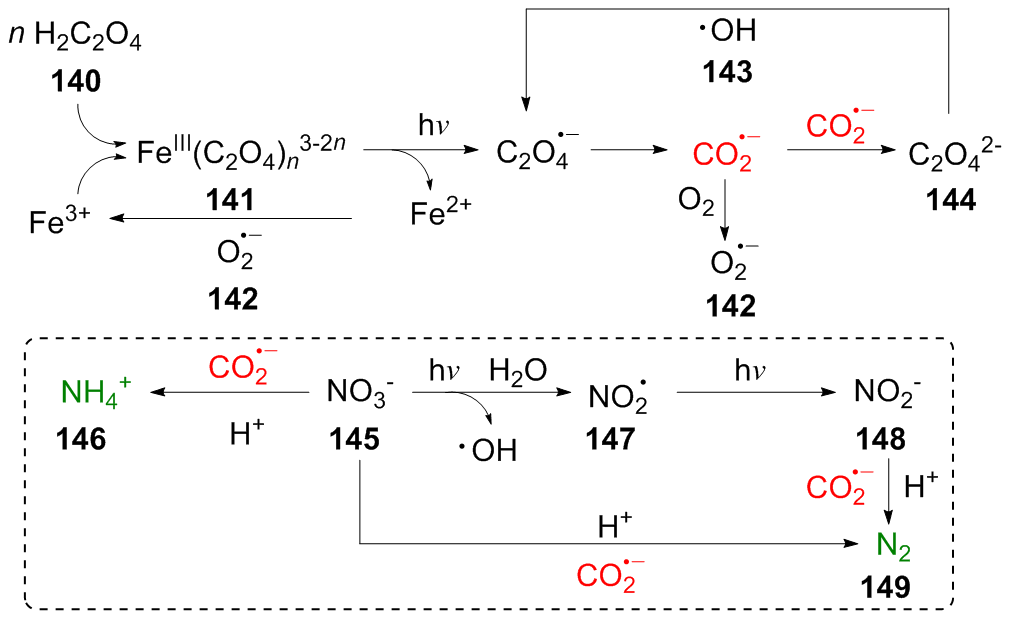
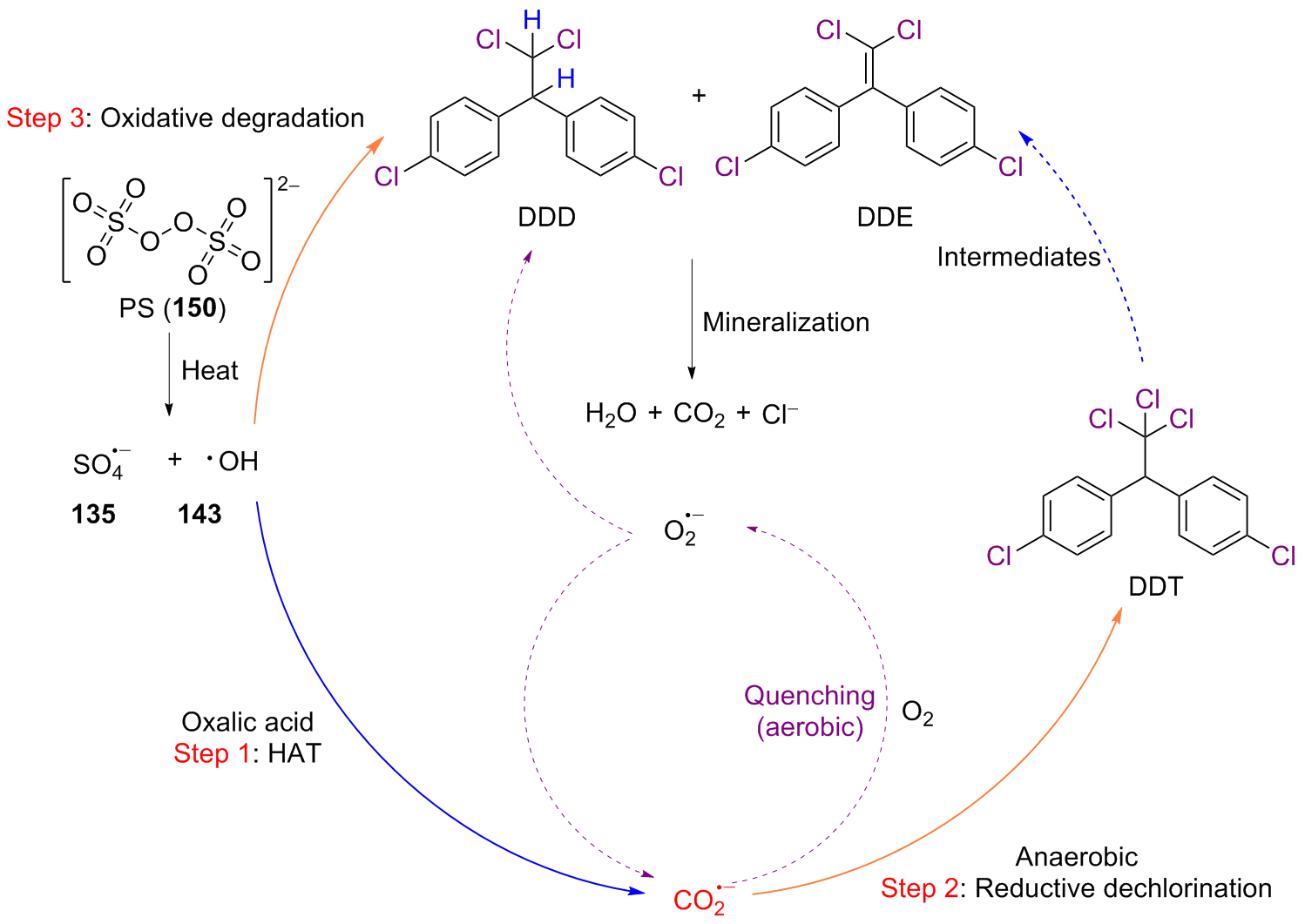
二氧化碳自由基阴离子($\mathrm{CO}_{2}^{-\cdot}$)是一种具有强还原性的活泼化学中间体, 其作为羧基来源和单电子还原剂在有机合成反应及污水还原降解中具有广泛的应用. CO2和甲酸及甲酸盐是常见的$\mathrm{CO}_{2}^{-\cdot}$来源, 能够通过单电子还原或氢原子转移过程转化为$\mathrm{CO}_{2}^{-\cdot}$. 本综述总结了$\mathrm{CO}_{2}^{-\cdot}$产生的机理及表征的方法, 介绍了$\mathrm{CO}_{2}^{-\cdot}$在各应用领域的最新研究进展, 并对$\mathrm{CO}_{2}^{-\cdot}$的应用前景提出了展望.
侯静, 黄燕, 李浩, 万远翠, 邵雨, 詹乐武, 王定海, 李斌栋. 二氧化碳自由基阴离子的应用研究进展[J]. 有机化学, 2024, 44(10): 3117-3135.
Jing Hou, Yan Huang, Hao Li, Yuancui Wan, Yu Shao, Lewu Zhan, Dinghai Wang, Bindong Li. Recent Advances in the Application of Carbon Dioxide Radical Anion[J]. Chinese Journal of Organic Chemistry, 2024, 44(10): 3117-3135.




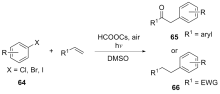
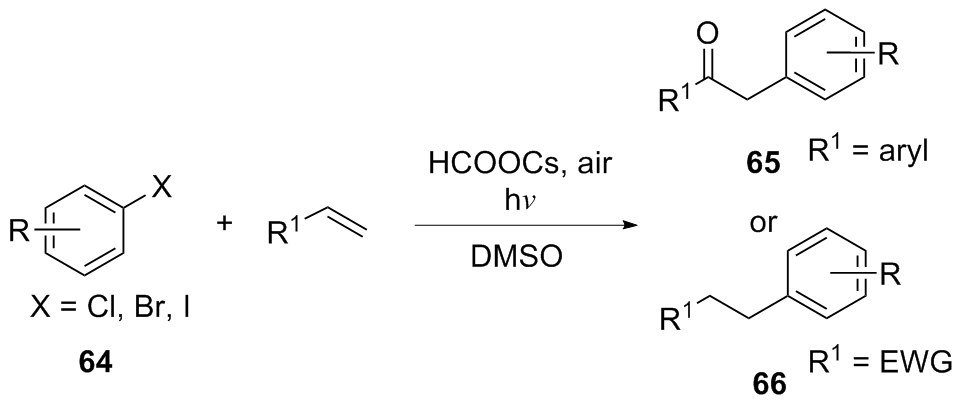
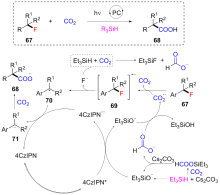
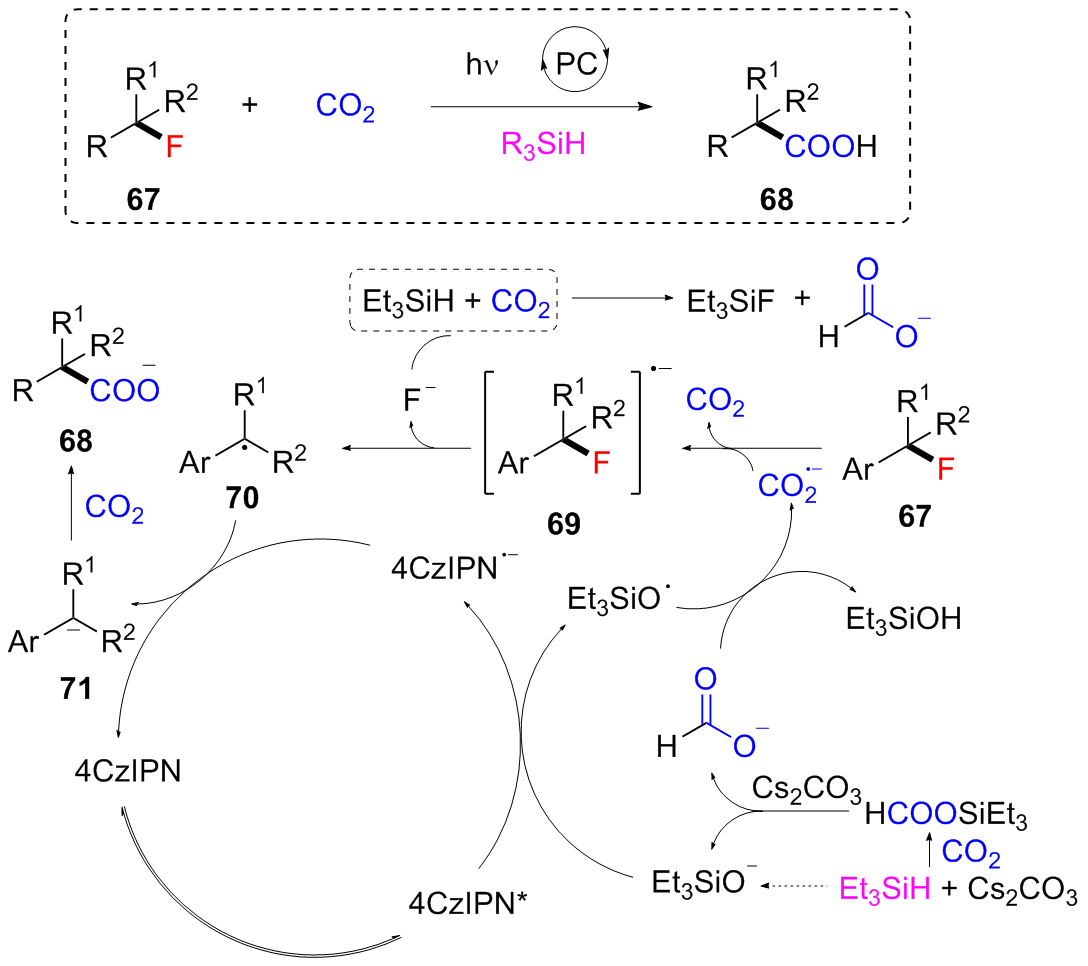
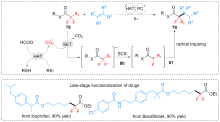
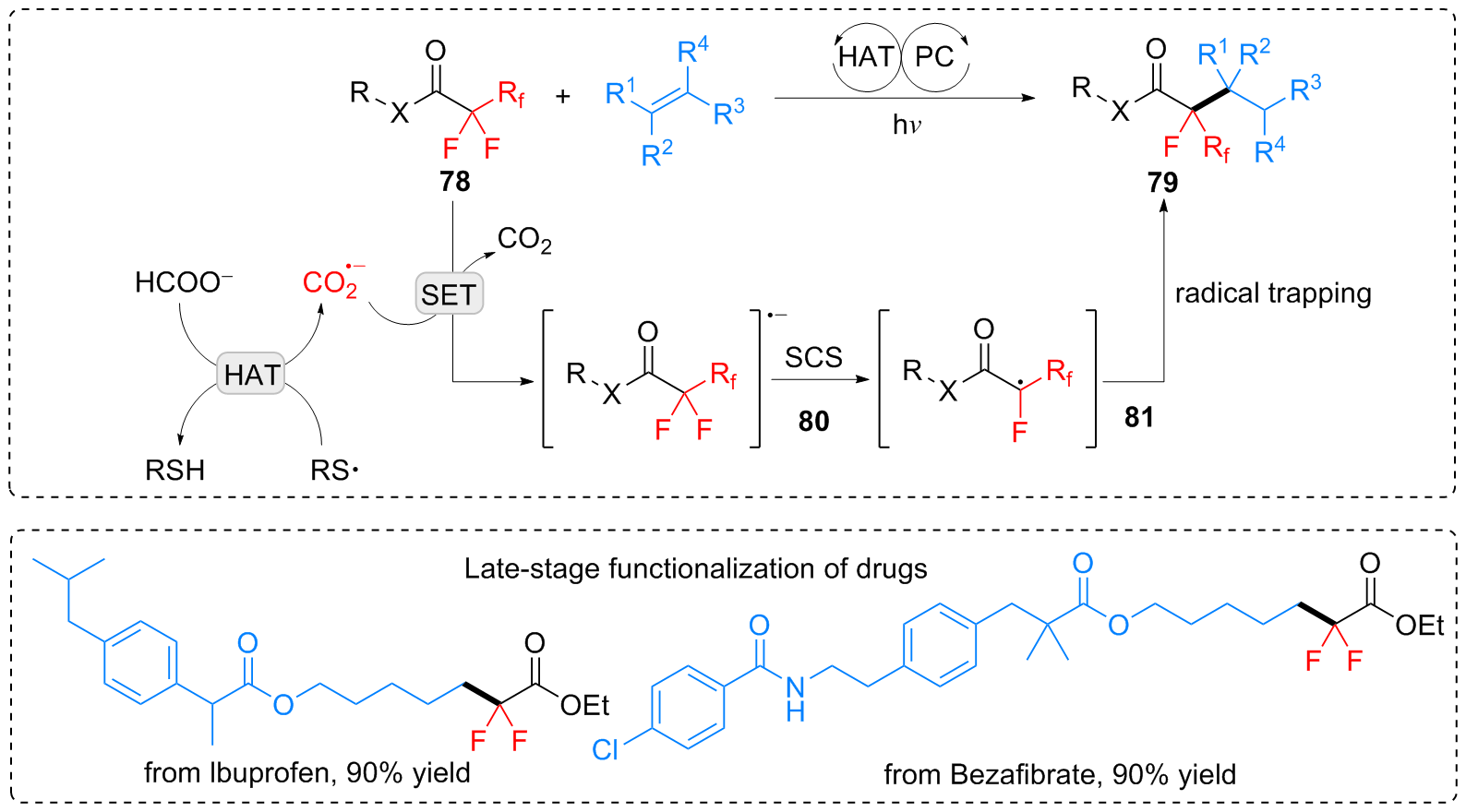
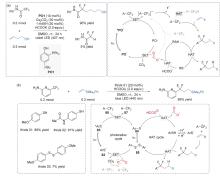

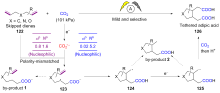

| [1] |
Koppenol, W. H.; Rush, J. D. J. Phys. Chem. 1987, 91, 4429.
|
| [2] |
Majhi, J.; Molander, G. A. Angew. Chem., Int. Ed. 2023, 63, e202311853.
|
| [3] |
Xiao, W.; Zhang, J.; Wu, J. ACS Catal. 2023, 13, 15991.
|
| [4] |
Gui, Y. Y.; Yan, S. S.; Yu, D. G. Sci. Bull. 2023, 68, 3124.
|
| [5] |
Rooso, J. A.; Bertolotti, S. G.; Braun, A. M. J. Phys. Org. Chem. 2001, 14, 300.
|
| [6] |
Arias-Rotondo, D. M.; Mccusker, J. K. Chem. Soc. Rev. 2016, 45, 5803.
pmid: 27711624 |
| [7] |
Ghosh, S.; Majumder, S.; Ghosh, D.; Hajra, A. Chem. Commun. 2023, 59, 7004.
|
| [8] |
Chalotra, N.; Kumar, J.; Naqvi, T.; Shah, B. A. Chem. Commun. 2021, 57, 11285.
|
| [9] |
Bao, X.; Yu, W.; Wang, G. Adv. Synth. Catal. 2023, 365, 2299.
|
| [10] |
Sivanandan, S. T.; Jesline, M. J.; Nair, D. K.; Kumar, T. Asian J. Org. Chem. 2023, 12, e202200555.
|
| [11] |
Matsumoto, A. J. Synth. Org. Chem., Jpn. 2022, 80, 868.
|
| [12] |
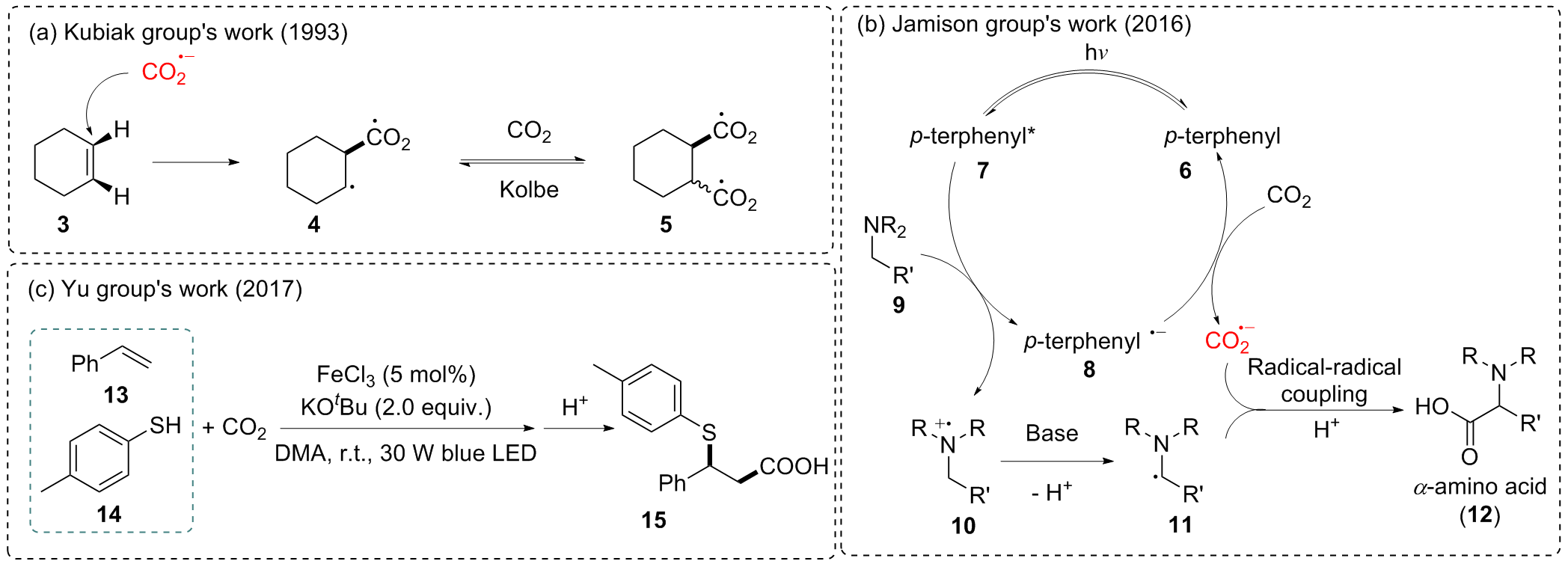
Morgenstern, D. A.; Wittrig, R. E.; Fanwick, P. E.; Kubiak, C. P. J. Am. Chem. Soc. 1993, 115, 6470.
|
| [13] |
Seo, H.; Katcher, M. H.; Jamison, T. F. Nat. Chem. 2017, 9, 453.
|
| [14] |
Seo, H.; Liu, A.; Jamison, T. F. J. Am. Chem. Soc. 2017, 139, 13969.
|
| [15] |
Ye, J. H.; Miao, M.; Huang, H.; Yan, S. S.; Yin, Z. B.; Zhou, W. J.; Yu, D. G. Angew. Chem., Int. Ed. 2017, 56, 15416.
|
| [16] |
Huang, H.; Ye, J. H.; Zhu, L.; Ran, C. K.; Miao, M.; Wang, W.; Chen, H. J.; Zhou, W. J.; Lan, Y.; Yu, B.; Yu, D. G. CCS Chem. 2020, 2, 1746.
|
| [17] |
Miao, M.; Zhu, L.; Zhao, H.; Song, L.; Yan, S. S.; Liao, L. L.; Ye, J. H.; Lan, Y.; Yu, D. G. Sci. China Chem. 2023, 66, 1457.
|
| [18] |
Zhang, W.; Chen, Z.; Jiang, Y. X.; Liao, L. L.; Wang, W.; Ye, J. H.; Yu, D. G. Nat. Commun. 2023, 14, 3529.
doi: 10.1038/s41467-023-39240-8 pmid: 37316537 |
| [19] |
Liao, L. L.; Wang, Z. H.; Cao, K. G.; Sun, G. Q.; Zhang, W.; Ran, C. K.; Li, Y. W.; Chen, L.; Cao, G. M.; Yu, D. G. J. Am. Chem. Soc. 2022, 144, 2062.
|
| [20] |
Zhang, W.; Liao, L. L.; Li, L.; Liu, Y.; Dai, L. F.; Sun, G. Q.; Ran, C. K.; Ye, J. H.; Lan, Y.; Yu, D. G. Angew. Chem., Int. Ed. 2023, 62, e202301892.
|
| [21] |
Yan, S. S.; Liu, S. H.; Chen, L.; Bo, Z. Y.; Jing, K.; Gao, T. Y.; Yu, B.; Lan, Y.; Luo, S. P.; Yu, D. G. Chem 2021, 7, 3099.
|
| [22] |
Sun, G. Q.; Yu, P.; Zhang, W.; Wang, Y.; Liao, L. L.; Zhang, Z.; Lu, Z. P.; Lin, S.; Yu, D. G. Nature 2023, 615, 67.
|
| [23] |
Wang, H.; Gao, Y. Z.; Zhou, C. L.; Li, G. J. Am. Chem. Soc. 2020, 142, 8122.
doi: 10.1021/jacs.0c03144 pmid: 32309942 |
| [24] |
Wang, H.; Jui, N. T. J. Am. Chem. Soc. 2018, 140, 163.
|
| [25] |
Vogt, D. B.; Seath, C. P.; Wang, H. B.; Jui, N. T. J. Am. Chem. Soc. 2019, 141, 13203.
|
| [26] |
Hendy, C. M.; Smith, G. C.; Xu, Z. H.; Lian, T. Q.; Jui, N. T. J. Am. Chem. Soc. 2021, 143, 8987.
|
| [27] |
Maust, C. M.; Hendy, C. M.; Jui, N. T.; Blakey, S. B. J. Am. Chem. Soc. 2022, 144, 3776.
doi: 10.1021/jacs.2c00192 pmid: 35200024 |
| [28] |
Alektiar, S. N.; Wickens, Z. K. J. Am. Chem. Soc. 2021, 143, 13022.
doi: 10.1021/jacs.1c07562 pmid: 34380308 |
| [29] |
Alektiar, S. N.; Han, J.; Dang, Y.; Rubel, C. Z.; Wickens, Z. K. J. Am. Chem. Soc. 2023, 145, 10991.
doi: 10.1021/jacs.3c03671 pmid: 37186951 |
| [30] |
Mikhael, M.; Alektiar, S. N.; Wickens, Z. K. Angew. Chem., Int. Ed. 2023, 62, e202303264.
|
| [31] |
Huang, Y.; Hou, J.; Zhan, L. W.; Zhang, Q.; Tang, W. Y.; Li, B. D. ACS Catal. 2021, 11, 15004.
|
| [32] |
Huang, Y.; Zhang, Q.; Zhan, L. W.; Hou, J.; Li, B. D. Chin. J. Org. Chem. 2022, 42, 2568. (in Chinese)
|
|
(黄燕, 张谦, 詹乐武, 侯静, 李斌栋, 有机化学, 2022, 42, 2568.)
doi: 10.6023/cjoc202202008 |
|
| [33] |
Huang, Y.; Zhang, Q.; Hua, L. L.; Zhan, L. W.; Hou, J.; Li, B. D. Cell Rep. Phys. Sci. 2022, 3, 100994.
|
| [34] |
Hou, J.; Hua, L. L.; Huang, Y.; Zhan, L. W.; Li, B. D. Chem. Asian J. 2022, e202201092.
|
| [35] |
Zhang, L. H.; Zhu, D.; Nathanson, G. M.; Hamers, R. J. Angew. Chem., Int. Ed. 2014, 53, 9746.
|
| [36] |
Khoshro, H.; Zare, H. R.; Vafazadeh, R. J. CO2 Util. 2015, 12, 77.
|
| [37] |
Ma, F.; Miao, T.; Zhou, Z. J.; Xu, H. L. RSC Adv. 2016, 6, 851.
|
| [38] |
Xu, J. Y.; Kan, Y. H.; Huang, R.; Zhang, B. S.; Wang, B.; Wu, K. H.; Lin, Y. M.; Sun, X. Y.; Li, Q. F.; Centi, G.; Su, D. S. ChemSus- Chem 2016, 9, 1085.
|
| [39] |
Lei, F. C.; Liu, W.; Sun, Y. F.; Xu, J. Q.; Liu, K.; Liang, L.; Yao, T.; Pan, B.; Wei, S.; Xie, Y. Nat. Commun. 2016, 7, 12697.
|
| [40] |
Mora, V. C.; Rosso, J. A.; Mártire, D. O.; Gonzalez, M. C.; Roux, G. C. L. Chemosphere 2009, 75, 1405.
|
| [41] |
Gu, X.; Lu, S.; Fu, X.; Qiu, Z. F.; Sui, Q.; Guo, X. H. Sep. Purif. 2017, 172, 211.
|
| [42] |
Gao, L.; Gu, X. G.; Lu, Z. H. China Environ. Sci. 2016, 36, 2645.
|
| [43] |
Tachikawa, T.; Tojo, S.; Fujitsuka, M.; Majima, T. Langmuir 2004, 20, 9441.
pmid: 15491173 |
| [44] |
Villamena, F. A.; Locigno, E. J.; Rockenbauer, A.; Hadad, C. M.; Zweier, J. L. J. Phys. Chem. A 2006, 110, 13253.
|
| [45] |
Berkovic, A. M.; Gonzalez, M. C.; Russo, N.; Michelini, M. C.; Diez, R. P.; Mártire, D. O. J. Phys. Chem. A 2010, 114, 12845.
doi: 10.1021/jp106035m pmid: 21086971 |
| [46] |
Subelzu, N.; Schöneich, C. Mol. Pharm. 2020, 17, 4163.
|
| [47] |
Zhang, Y. L.; Richards, D. S.; Grotemeyer, E. N.; Jackson, T. A.; Schöneich, C. Mol. Pharm. 2022, 19, 4026.
|
| [48] |
Amatore, C.; Savéant, J. M. J. Am. Chem. Soc. 1981, 103, 5021.
|
| [49] |
Matsuoka, S.; Kohzuki, T.; Yanagida, S.; Pac, C.; Ishida, A.; Takamuku, S.; Kusaba, M.; Nakashima, N.; Yanagida, S. J. Phys. Chem. B 1992, 96, 4437.
|
| [50] |
Meerholz, K.; Heinze, J. J. Am. Chem. Soc. 1989, 111, 2326.
|
| [51] |
Isse, A. A.; Gennaro, A. Chem. Commun. 2002, 2798.
|
| [52] |
Kai, T.; Zhou, M.; Duan, Z. Y.; Henkelman, G. A.; Bard, A. J. J. Am. Chem. Soc. 2017, 139, 18552.
|
| [53] |
Lamy, E.; Nadjo, L.; Saveant, J. M. J. Electroanal. Chem. 1977, 78, 403.
|
| [54] |
Hammouche, M.; Lexa, D.; Momenteau, M.; Savéant, J. M. J. Am. Chem. Soc. 1991, 113, 8455.
|
| [55] |
Ito, T.; Hata, H.; Nishimoto, S. Int. J. Radiat. 2000, 76, 683.
|
| [56] |
Eugenio, S. M.; Clifford, P. K. Organometallics 2005, 24, 96.
|
| [57] |
Li, J.; Inagi, S.; Fuchigami, T.; Hosono, H.; Ito, S. Electrochem. Commun. 2014, 44, 45.
|
| [58] |
Liu, X. W.; Zhong, J.; Fang, L.; Wang, L. L.; Ye, M.; Shao, Y.; Li, J.; Zhang, T. Chem. Eng. J. 2016, 303, 56.
|
| [59] |
Qin, B. Y.; Tang, H.; Xu, J. P. China Environ. Sci. 2018, 38, 2505.
|
| [60] |
Li, B. Z. Shanghai Environ. Sci. 2019, 38, 47.
|
| [61] |
Grills, D. C.; Lymar, S. V. Phys. Chem. Chem. Phys. 2018, 20, 10011.
doi: 10.1039/c8cp00977e pmid: 29620127 |
| [62] |
Ju, T.; Zhou, Y. Q.; Cao, K. G.; Fu, Q.; Ye, J. H.; Sun, G. S.; Liu, X. F., Chen, L.; Liao, L. L.; Yu, D. G. Nat. Catal. 2021, 4, 304.
|
| [63] |
Song, L.; Wang, W.; Yue, J. P.; Jiang, Y. X.; Wei, M. K.; Zhang, H. P.; Yan, S. S.; Liao, L. L.; Yu, D. G. Nat. Catal. 2022, 5, 832.
|
| [64] |
Zhou, C. L.; Wang, X. C.; Yang, L.; Fu, L.; Li, G. Green Chem. 2022, 24, 6100.
|
| [65] |
Fu, M. C.; Wang, J. X.; Ge, W.; Du, F. M.; Fu, Y. Org. Chem. Front. 2023, 10, 35.
|
| [66] |
Xu, P.; Wang, S.; Liu, Y. Q.; Li, R. B.; Liu, W. W.; Wang, X. P.; Zou, M. L.; Zhou, Y.; Guo, D.; Zhu, X. ACS Catal. 2023, 13, 2149.
|
| [67] |
Zhang, F. L.; Wu, X. Y.; Gao, P. P.; Zhang, H.; Li, Z.; Ai, S.; Li, G. Chem. Sci. 2024, 15, 6178.
|
| [68] |
Mangaonkar, S. R.; Hayashi, H.; Takano, H.; Kanna, W.; Maeda, S.; Mita, T. ACS Catal. 2023, 13, 2482.
|
| [69] |
Wu, Z. G.; Wu, M. Y.; Zhu, K.; Wu, J.; Lu, Y. X. Chem 2023, 9, 978.
|
| [70] |
Alyah, F. C.; Oliver, P. W.; Chernowsky, C. P.; Yeung, C. S.; Wickens, Z. K. J. Am. Chem. Soc. 2021, 143, 10882.
|
| [71] |
Campbell, M. W.; Polites, V. C.; Patel, S.; Lipson, J. E.; Majhi, J.; Molander, G. A. J. Am. Chem. Soc. 2021, 143, 19648.
doi: 10.1021/jacs.1c11059 pmid: 34793157 |
| [72] |
Ye, J. H.; Bellotti, P.; Heusel, C.; Glorius, F. Angew. Chem., Int. Ed. 2022, 61, e202115456.
|
| [73] |
Liu, C.; Shen, N.; Shang, R. Nat. Commun. 2022, 13, 354.
|
| [74] |
Liu, C.; Li, K.; Shang, R. ACS Catal. 2022, 12, 4103.
|
| [75] |
Xu, P.; Wang, X. Y.; Wang, Z. J.; Zhao, J. J.; Cao, X. D.; Xiong, X. C.; Yuan, Y. C.; Zhu, S.; Guo, D.; Zhu, X. Org. Lett. 2022, 24, 4075.
|
| [76] |
Hendy, C. M.; Pratt, C. J.; Jui, N. T.; Blakey, S. B. Org. Lett. 2023, 25, 1397.
|
| [77] |
Saini, V.; Stokes, B. J.; Sigman, M. S. Angew. Chem., Int. Ed. 2013, 52, 11206.
|
| [78] |
Juliá, F.; Yan, J.; Paulus, F.; Ritter, T. J. Am. Chem. Soc. 2021, 143, 12992.
|
| [79] |
Yu, J. J.; Zhang, X.; Wu, X.; Zhang, Z. Q.; Wu, J.; Zhu, C. Chem 2023, 9, 472.
|
| [80] |
Huang, Y.; Wan, Y. C.; Shao, Y.; Zhan, L. W.; Hou, J.; Li, B. D. Green Chem. 2023, 25, 8280.
|
| [81] |
Herburger, A.; Oncak, M.; Barwa, E.; Linde, C.V.; Beyer, M. K. Int. J. Mass Spectrom. 2019, 435, 101.
doi: 10.1016/j.ijms.2018.10.019 pmid: 33209089 |
| [82] |
Cencer, M. M.; Li, C. Y.; Agarwal, G.; Neto, R. J.; Amanchukwu, C.V.; Assary, R. S. ACS Omega 2022, 7, 18131.
|
| [83] |
Alkayal, A.; Tabas, V.; Montanaro, S.; Wright, L. A.; Malkov, A. V.; Buckley, B. R. J. Am. Chem. Soc. 2020, 142, 1780.
doi: 10.1021/jacs.9b13305 pmid: 31960672 |
| [84] |
You, Y.; Kanna, W.; Mita, T. J. Am. Chem. Soc. 2022, 144, 3685.
|
| [85] |
Zhao, L.; Xie, W. J.; Li, H. R.; He, L. N. Org. Lett. 2024, 26, 3241.
doi: 10.1021/acs.orglett.4c00860 pmid: 38578088 |
| [86] |
Zhang, K.; Ren, B. H.; Liu, X. F.; Wang, L. L.; Zhang, M.; Ren, W. M.; Lu, X. B.; Zhang, W. Z. Angew. Chem., Int. Ed. 2022, 61, e202207660.
|
| [87] |
Wang, Y. W.; Tang, S. Y.; Qiu, Y. A. Angew. Chem., Int. Ed. 2022, 61, e202207746.
|
| [88] |
Ran, C.-K.; Qu, Q.; Tao, Y.-Y.; Chen, Y.-F.; Liao, L.-L.; Ye, J.-H.; Yu, D.-G. Sci. China Chem. 2024, 67, 3366.
|
| [89] |
Rosso, J. A.; Bertolotti, S. G.; Braun, A. M.; Mártire, D. O.; Gonzalez, M. C. J. Phys. Org. Chem. 2001, 14, 300.
|
| [90] |
Tachikawa, T.; Tojo, F. M.; Fujitsuka, M.; Majima, T. Langmuir 2004, 20, 9441.
pmid: 15491173 |
| [91] |
Schutz, O.; Meyerstein, D. Tetrahedron Lett. 2006, 47, 1093.
|
| [92] |
Mora, V. C.; Ross, J. A.; Carrillo, L. R.; Mártire, D. O.; Gonzalez, M. C. Chemosphere 2009, 75, 1405.
|
| [93] |
Liu, X. W.; Fang, L.; Wang, L. L.; Ye, M. M.; Shao, Y.; Li, J.; Zhang, T. Q. Chem. Eng. J. 2016, 303, 56.
|
| [94] |
Wu, W. M.; Liu, G. D.; Liang, S. J.; Chen, Y.; Shen, L. J.; Zhen, H. R.; Yuan, R. S.; Hou, Y. D.; Wu, L. J. Catal. 2012, 290, 13.
|
| [95] |
Liu, Y. X.; Wang, L.; Wu, F.; Deng, N. S. Desalin. Water Treat. 2013, 51, 7194.
|
| [96] |
Liu, X. W.; Zhong, J. E.; Fang, L.; Wang, L. L.; Ye, M. M.; Shao, Y.; Li, J.; Zhang, T. Q. Chem. Eng. J. 2016, 303, 56.
|
| [97] |
Ren, H. J.; Hou, Z. M.; Hou, Z. M.; Ren, H. J.; Hou, Z. M.; Han, X.; Zhou, R. Chem. Eng. J. 2017, 309, 638.
|
| [98] |
Amina; Si, X. Y.; Wu, K.; Si, Y. B.; Yousaf, B. Chem. Eng. J. 2020, 384, 123360.
|
| [99] |
AlSalka, Y.; Al-Madanat, O.; Curti, M.; Hakki, A.; Bahnemann, D. W. ACS Appl. Energy Mater. 2020, 3, 6678.
|
| [100] |
Prajapati, I.; Subelzu, N.; Zhang, Y. L.; Wu, Y. Q.; Schöneich, C. J. Pharm. Sci. 2022, 111, 991.
|
| [101] |
Shi, Z. Y.; Wang, F. L.; Xiao, Q.; Yu, S. L.; Ji, X. L. Catalysts 2022, 12, 348.
|
| [102] |
Qu, J. H.; Tian, X.; Zhang, X. B.; Yao, J. Y.; Xue, J. Q.; Li, K. G.; Zhang, B.; Zhang, Y. Appl. Catal., B 2022, 310, 121359.
|
| [1] | 陈亮, 胡良建, 杜宇, 苏伟平, 康强. 中心手性金属铑配合物催化的不对称光诱导Giese自由基加成反应[J]. 有机化学, 2020, 40(11): 3944-3952. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||