有机化学 ›› 2025, Vol. 45 ›› Issue (1): 246-255.DOI: 10.6023/cjoc202406010 上一篇 下一篇
研究论文
王浩洋, 成琳, 曾星, 韩利民, 杜玉英*( ), 竺宁*(
), 竺宁*( )
)
收稿日期:2024-06-08
修回日期:2024-08-03
发布日期:2024-08-30
基金资助:
Haoyang Wang, Lin Cheng, Xing Zeng, Limin Han, Yuying Du( ), Ning Zhu(
), Ning Zhu( )
)
Received:2024-06-08
Revised:2024-08-03
Published:2024-08-30
Contact:
*E-mail: Supported by:文章分享

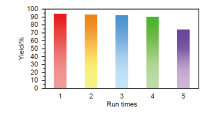
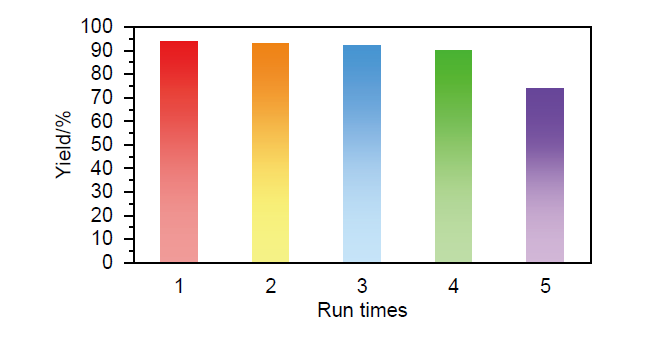
以功能化离子液体氢氧化1-丁基-3-甲基咪唑([BMim]OH)为催化剂, 由α-卤代物与亚磺酸钠盐通过偶联反应构建了β-酮砜和砜类化合物的绿色合成方法. 在体积分数为50%的乙醇水溶液中反应2~6 h, 以65%~99%的产率获得34种不同的β-酮砜和砜. 此外, 该反应可以放大至克级规模, 并且[BMim]OH催化剂可以循环使用至少4次, 产率无明显下降. 机理研究表明, 反应过程中催化量的[BMim]OH首先与苯亚磺酸根(PhSO2-)作用形成中间体1-丁基-3-甲基咪唑苯亚磺酸盐([BMim][PhSO2]), 之后利用中间体硫负离子的强亲核性与α-溴代苯乙酮反应, 从而促进该SN2反应的进行. 此方法具有操作简便、产率高、官能团兼容性好、无需使用金属催化剂及对环境友好等优点.
王浩洋, 成琳, 曾星, 韩利民, 杜玉英, 竺宁. 功能化离子液体[BMim]OH催化α-卤代物与亚磺酸钠盐合成β-酮砜及砜类化合物[J]. 有机化学, 2025, 45(1): 246-255.
Haoyang Wang, Lin Cheng, Xing Zeng, Limin Han, Yuying Du, Ning Zhu. Functionalized Ionic Liquid [BMim]OH-Catalyzed the Synthesis of β-Ketosulfones and Sulfone Compounds between α-Halides and Sodium Sulfinate[J]. Chinese Journal of Organic Chemistry, 2025, 45(1): 246-255.
| Entry | 1a∶2a | [BMim]OHa/% | T/℃ | Solvent | Time/h | Yieldb/% |
|---|---|---|---|---|---|---|
| 1 | 1∶1 | — | r.t. | Ethanol (φ=50%) | 2 | 45 |
| 2 | 1∶1 | 20 | r.t. | Ethanol (φ=50%) | 2 | 68 |
| 3 | 1∶1.2 | 20 | r.t. | Ethanol (φ=50%) | 2 | 79 |
| 4 | 1∶1.5 | 20 | r.t. | Ethanol (φ=50%) | 2 | 94 (80c) |
| 5 | 1∶2 | 20 | r.t. | Ethanol (φ=50%) | 2 | 95 |
| 6 | 1∶1.5 | 5 | r.t. | Ethanol (φ=50%) | 2 | 79 |
| 7 | 1∶1.5 | 10 | r.t. | Ethanol (φ=50%) | 2 | 86 |
| 8 | 1∶1.5 | 15 | r.t. | Ethanol (φ=50%) | 2 | 89 |
| 9 | 1∶1.5 | 30 | r.t. | Ethanol (φ=50%) | 2 | 95 |
| 10 | 1∶1.5 | 20 | 60 | Ethanol (φ=50%) | 1.5 | 98 |
| 11 | 1∶1.5 | 20 | r.t. | H2O | 2 | 60 |
| 12 | 1∶1.5 | 20 | r.t. | Anhydrous ethanol | 2 | 65 |
| Entry | 1a∶2a | [BMim]OHa/% | T/℃ | Solvent | Time/h | Yieldb/% |
|---|---|---|---|---|---|---|
| 1 | 1∶1 | — | r.t. | Ethanol (φ=50%) | 2 | 45 |
| 2 | 1∶1 | 20 | r.t. | Ethanol (φ=50%) | 2 | 68 |
| 3 | 1∶1.2 | 20 | r.t. | Ethanol (φ=50%) | 2 | 79 |
| 4 | 1∶1.5 | 20 | r.t. | Ethanol (φ=50%) | 2 | 94 (80c) |
| 5 | 1∶2 | 20 | r.t. | Ethanol (φ=50%) | 2 | 95 |
| 6 | 1∶1.5 | 5 | r.t. | Ethanol (φ=50%) | 2 | 79 |
| 7 | 1∶1.5 | 10 | r.t. | Ethanol (φ=50%) | 2 | 86 |
| 8 | 1∶1.5 | 15 | r.t. | Ethanol (φ=50%) | 2 | 89 |
| 9 | 1∶1.5 | 30 | r.t. | Ethanol (φ=50%) | 2 | 95 |
| 10 | 1∶1.5 | 20 | 60 | Ethanol (φ=50%) | 1.5 | 98 |
| 11 | 1∶1.5 | 20 | r.t. | H2O | 2 | 60 |
| 12 | 1∶1.5 | 20 | r.t. | Anhydrous ethanol | 2 | 65 |
| |
| |
| Entry | Catalyst | Condition | Time | Yield/% | Ref. |
|---|---|---|---|---|---|
| 1 | KI | V(AcOH)∶V(DMSO)=1∶1, 80 ℃ | 12 h | 57 (3a) | [ |
| 2 | CuBr2 | DMSO, r.t. | 1 h | 85 (3a) | [ |
| 3 | Free Cat. | V(C2H5OH)∶V(H2O)=3∶1, 50 ℃ | 10 h | 65 (3m) | [ |
| 4 | Mn, I2 | DMA, r.t. | 24 h | 80 (3a) | [ |
| 5 | Cu25%/ZIF-8 | MeOH, r.t. | 12 h | 89 (3m) | [ |
| 6 | Et3N, I2 | H2O | 10 min | 85 (3a) | [ |
| 7 | PG(+)|St(-), KI, TBHP | MeOH, r.t. | 10 h | 82 (3a) | [ |
| 8 | [BMim]OH | V(EtOH)∶V(H2O)=1∶1, r.t. | 2 h | 94 (3a) | This work |
| Entry | Catalyst | Condition | Time | Yield/% | Ref. |
|---|---|---|---|---|---|
| 1 | KI | V(AcOH)∶V(DMSO)=1∶1, 80 ℃ | 12 h | 57 (3a) | [ |
| 2 | CuBr2 | DMSO, r.t. | 1 h | 85 (3a) | [ |
| 3 | Free Cat. | V(C2H5OH)∶V(H2O)=3∶1, 50 ℃ | 10 h | 65 (3m) | [ |
| 4 | Mn, I2 | DMA, r.t. | 24 h | 80 (3a) | [ |
| 5 | Cu25%/ZIF-8 | MeOH, r.t. | 12 h | 89 (3m) | [ |
| 6 | Et3N, I2 | H2O | 10 min | 85 (3a) | [ |
| 7 | PG(+)|St(-), KI, TBHP | MeOH, r.t. | 10 h | 82 (3a) | [ |
| 8 | [BMim]OH | V(EtOH)∶V(H2O)=1∶1, r.t. | 2 h | 94 (3a) | This work |
| [1] |
(a) Pal, T. K.; Dey, S.; Pathak, T. J. Org. Chem. 2011, 76, 3034.
|
|
(b) Xu, W. M.; Han, F.-F.; He, M.; Hu, D. Y.; He, J.; Yang, S.; Song, B. A. J. Agric. Food Chem. 2012, 60, 1036.
|
|
|
(c) Zhang, B.; Wassermann, A. M.; Vogt, M.; Bajorath, J. J. Chem. Inf. Model. 2012, 52, 3138.
|
|
|
(d) Consalvi, S.; Alfonso, S.; Di Capua, A.; Poce, G.; Pirolli, A.; Sabatino, M.; Ragno, R.; Anzini, M.; Sartini, S.; La Motta, C.; Di Cesare, M. L.; Ghelardini, C.; Biava, M. Bioorg. Med. Chem. 2015, 23, 810.
|
|
| [2] |
(a) Aranapakam, V.; Grosu, G. T.; Davis, J. M.; Hu, B.; Ellingboe, J.; Baker, J. L.; Skotnicki, J. S.; DiJoseph, J. F.; Sung, A.; Sharr, M. A.; Killar, L. M.; Walter, T.; Jin, G. X.; Cowling, R. J. Med. Chem. 2003, 46, 2361.
|
|
(b) Peng, H.; Cheng, Y.; Ni, N.; Li, M.; Choudhary, G.; Chou, H. T.; Lu, C. D.; Tai, P. C.; Wang, B. ChemMedChem 2009, 4, 1457.
|
|
|
(c) Abdel-Aziz, H. A.; Al-Rashood, K. A.; ElTahir, K. E. H.; Suddek, G. M. Eur. J. Med. Chem. 2014, 80, 416.
|
|
|
(d) Zheng, M.; Li, G.; Lu, H. Org. Lett. 2019, 21, 1216.
|
|
| [3] |
Yalavarthi, N. R.; Gundoju, N.; Bokam, R.; Ponnapalli, M. G. J. Chem. Sci. 2019, 131.
|
| [4] |
Han, F.; Su, B.; Song, P.; Wang, Y.; Jia, L.; Xun, S.; Hu, M.; Zou, L. Tetrahedron 2018, 74, 5908.
|
| [5] |
Yavari, I.; Shaabanzadeh, S. Org. Lett. 2020, 22, 464.
|
| [6] |
Xu, J.; Shen, C.; Qin, X.; Wu, J.; Zhang, P.; Liu, X. J. Org. Chem. 2021, 86, 3706.
|
| [7] |
Wang, Y. J.; Zhao, Y. H.; Cai, C. Q.; Wang, L. Y.; Gong, H. Org. Lett. 2021, 23, 8296.
|
| [8] |
Pampana, V. K. K.; Charpe, V. P.; Sagadevan, A.; Das, D. K.; Lin, C.-C.; Hwu, J. R.; Hwang, K. C. Green Chem. 2021, 23, 3569.
|
| [9] |
Fang, Y.; Xu, D. P.; Yu, Y. L.; Tang, R. M.; Dai, S.-S.; Wang, G. H. Eur. J. Org. Chem. 2022, 13, 1.
|
| [10] |
Song, Y. L.; Wang, X. C.; Huang, C. P.; Liang, F. B.; Yu, Z. C.; Chen, B. H. Chin. J. Org. Chem. 2013, 33, 1715 (in Chinese).
|
|
(宋彦磊, 王新承, 黄崇品, 梁凤兵, 毓志超, 陈标华, 有机化学, 2013, 33, 1715.)
|
|
| [11] |
(a) Lourenço, N. M. T.; Afonso, C. A. M. Tetrahedron 2003, 59, 789.
|
|
(b) Jorapur, Y. R.; Chi, D. Y. Bull. Korean Chem. Soc. 2006, 27, 345.
|
|
|
(c) Zhao, D. W.; Wu, Y. T.; Chen, T.-T.; Dai, L. Y.; Wang, Y.-Y. Chin. J. Org. Chem. 2013, 33, 1791 (in Chinese).
|
|
|
(赵东旺, 吴悦彤, 陈婷婷, 戴立益, 王媛媛, 有机化学, 2013, 33, 1791.)
|
|
| [12] |
(a) Hu, Y.; Chen, Z. C.; Le, Z. G.; Zheng, Q. G. J. Chem. Res. 2004, 4, 267.
|
|
(b) Li, J. X.; Yang, S. R.; Wu, W. Q.; Jiang, H. F. Eur. J. Org. Chem. 2018, 1284.
|
|
|
(c) Lai, Y. L.; Wu, L. Y.; Xiong, X.; Lan, Y. W.; Lin, Y.-Y.; Zhong, R. M.; Jiang, H. F.; Li, J. X. Green Synth. Catal.DOI: 10.1016/j.gresc.2024.02.003.
|
|
| [13] |
(a) Suryakiran, N.; Prabhakar, P.; Rajesh, K.; Suresh, V.; Venkateswarlu, Y. J. Mol. Catal. A: Chem. 2007, 270, 201.
|
|
(b) Hu, M.; Lin, Z. D.; Li, J. X.; Wu, W. Q.; Jiang, H. F. Green Chem. 2020, 22, 5584.
|
|
|
(c) Li, J. X.; He, D.; Lin, Z. D.; Cen, L. Y.; Wu, W. Q.; Jiang, H. F. Green Chem. 2022, 24, 1983.
|
|
| [14] |
Kumar, A.; Muthyala, M. K. Tetrahedron Lett. 2011, 52, 5368.
|
| [15] |
Moulton, R. US 20030094380, 2003.
|
| [16] |
Yuen, A. K. L.; Masters, A. F.; Maschmeyer, T. Catal. Today 2013, 200, 9.
|
| [17] |
Zhang, H. L.; Li, M. Y.; Wang, N. Appl. Chem. Ind. 2013, 42, 1866 (in Chinese).
|
|
(张海龙, 李梦耀, 王娜, 应用化工, 2013, 42, 1866.)
|
|
| [18] |
Song, Z.; Wang, H.; Xing, L. J. Solution Chem. 2009, 38, 1139.
|
| [19] |
Zhao, P.-P.; Hu, K.; Cai, P.; Cheng, G. Z. Univ. Chem. 2022, 37, 2208150 (in Chinese).
|
|
(赵苹苹, 胡锴, 蔡苹, 程功臻, 大学化学, 2022, 37, 2208150.)
|
|
| [20] |
Ye, M. F.; Cao, Y. Z.; Ding, R. H.; Lei, Z. P.; Jin, L.; Zhang, J. Chem. Ind. Times 2019, 33, 9 (in Chinese).
|
|
(叶明富, 曹云钟, 丁仁浩, 雷智平, 金玲, 张婧, 化工时刊, 2019, 33, 9.)
|
|
| [21] |
Liu, L.; Xiao, H.; Xiao, F. H.; Xie, Y. J.; Huang, H. W.; Deng, G. J. Chin. J. Org. Chem. 2021, 41, 4749 (in Chinese).
|
|
(刘丽, 肖洪, 肖福红, 谢艳军, 黄华文, 邓国军, 有机化学, 2021, 41, 4749.)
|
|
| [22] |
Xu, L.; Lv, L.-L.; Wang, X. S. Chin. J. Org. Chem. 2023, 43, 3644 (in Chinese).
|
|
(许力, 吕兰兰, 王香善, 有机化学, 2023, 43, 3644.)
|
|
| [23] |
Gulizabair, A. M.D. Dissertation, Xinjiang Normal University, Xinjiang, 2021 (in Chinese).
|
|
(古丽扎拜尔•阿不力皮孜, 硕士论文, 新疆师范大学, 新疆, 2021.)
|
|
| [24] |
Chen, Z.; Guo, K.; Chen, R. S.; Gu, C.; Zhou, H. T.; Zhu, Y. G. Chin. J. Org. Chem. 2018, 38, 963 (in Chinese).
|
|
(陈震, 郭康, 陈荣顺, 顾晨, 周华婷, 朱映光, 有机化学, 2018, 38, 963.)
|
|
| [25] |
He, Y.; Yin, H. G.; Zeng, Z. W.; Zeng, H. T. Shandong Chem. Ind. 2022, 51, 8 (in Chinese).
|
|
(何燕, 尹红果, 曾智文, 曾慧婷, 山东化工, 2022, 51, 8.)
|
|
| [26] |
(a) Liu, C. R.; Ding, L. H.; Guo, G.; Liu, W.-W. Eur. J. Org. Chem. 2016, 2016, 910.
|
|
(b) Suryakiran, N.; Reddy, T. S.; Ashalatha, K.; Lakshman, M.; Venkateswarlu, Y. Tetrahedron. Lett. 2006, 47, 3853.
|
|
| [27] |
(a) Rawat, V. S.; Reddy, P. L. M.; Sreedhar, B. RSC. Adv. 2014, 4, 5165.
|
|
(b) Tang, X.; Huang, L.; Xu, Y.; Yang, J.; Wu, W.; Jiang, H. Angew. Chem., Int. Ed. 2014, 53, 4205.
|
|
| [28] |
(a) Yang, J.; Li, H. Q.; Li, M. H.; Peng, J.-J.; Gu, Y. L. Adv. Synth. Catal. 2012, 354, 688.
|
|
(b) Chang, M. Y.; Cheng, Y. C.; Lu, Y. J. Org. Lett. 2014, 16, 6252.
|
|
| [29] |
(a) Fu, J.; Li, Q. Z.; Li, M. P.; Du, Z. Y. ChemistrySelect 2020, 5, 2985.
|
|
(b) Chen, X. Y.; Lu, S. X.; Zheng, Y.-Y.; Wang, J. G.; Yang, L.; Sun, P. Adv. Synth. Catal. 2022, 364, 1305.
|
|
| [30] |
(a) Wagh, G. D.; Autade, S. B.; Kulkarni, R. V.; Akamanchi, K. G. New J. Chem. 2020, 44, 10554.
|
|
(b) Truitt, P.; Stead, R.; Long, L. M.; Middleton, W. J. J. Am. Chem. Soc. 1949, 71, 3511.
|
|
| [31] |
Jiang, H.; Cheng, Y.; Zhang, Y.; Yu, S. Eur. J. Org. Chem. 2013, 2013, 5485.
|
| [32] |
(a) Xiong, Y. H.; Weng, J.; Lu, G. Adv. Synth. Catal. 2018, 360, 1611.
|
|
(b) Lu, Q.; Zhang, J.; Zhao, G.; Qi, Y.; Wang, H.; Lei, A. J. Am. Chem. Soc. 2013, 135, 11481.
|
|
| [33] |
Tsui, G. C.; Glenadel, Q.; Lau, C.; Lautens, M. Org. Lett. 2011, 13, 208.
|
| [34] |
Yuan, Z. Y.; Zhang, S.; Teng, F.; Jin, X. F.; Sheng, W. B.; Gui, Q. W. ChemistrySelect 2022, 7, e202103355.
|
| [35] |
(a) Reddy, R. J.; Kumar, J.-J.; Kumari, A. H. Eur. J. Org. Chem. 2019, 23, 3771.
|
|
(b) Jeyakumar, K.; Chand, D. K. Tetrahedron Lett. 2006, 47, 4573.
|
|
|
(c) Wang, Z.; Jiang, J.; Pang, S.; Zhou, Y.; Guan, C.; Gao, Y.; Li, J.; Yang, Y.; Qiu, W.; Jiang, C. C. Environ. Sci. Technol. 2018, 52, 11276.
|
|
| [36] |
Wildeman, J.; Van Leusen, A. M. Synthesis 1979, 1979, 733.
|
| [37] |
Karimi, B.; Khorasani, M. ACS. Catal. 2013, 3, 1657.
|
| [38] |
Zhang, F. X.; Zhou, F. X.; Yin, S. H.; Long, B.; Deng, G. J.; Ali, A.; Song, T. Appl. Catal., B 2023, 337, 123004.
|
| [39] |
Onanong, V.; Khanchyd, M.; Praewpan, K.; Chutima, K.; Chutima, J. Bioorg. Med. Chem. Lett. 2022, 63, 128652
|
| [1] | 王晓琴, 许盛, 平媛媛, 孔望清. 光/镍协同催化实现C(sp3)—H键选择性官能团化[J]. 有机化学, 2025, 45(2): 383-422. |
| [2] | 梅明顺, 张扬会. 钯催化惰性亚甲基C(sp3)—H键分子间官能团化反应[J]. 有机化学, 2025, 45(2): 620-640. |
| [3] | 王曼曼, 习文慧, 吴昊, 白大昌. 过渡金属催化碳氢键活化合成烷基氟烷基化合物的研究进展[J]. 有机化学, 2025, 45(2): 516-530. |
| [4] | 李永梅, 孙亮博, 徐坤, 曾程初. 基于2,3-二氯-5,6-二氰基-1,4-苯醌(DDQ)的电/光电催化在碳-氢和碳-氟键官能化中的应用进展[J]. 有机化学, 2025, 45(2): 668-676. |
| [5] | 沈佳斌, 沈超, 章鹏飞. 可见光介导的羰基α位C—H官能团化反应合成萘咪酮类衍生物[J]. 有机化学, 2025, 45(2): 677-685. |
| [6] | 林恩泽, 李必杰. 基于碳氢键断裂的金属催化的内烯烃不对称氢芳基化进展[J]. 有机化学, 2025, 45(2): 546-558. |
| [7] | 张朝威, 徐兵斌, 刘文龙, 赵敬, 段伟良. 钯催化不对称碳氢键活化合成平面手性二茂铁磺酰胺化合物[J]. 有机化学, 2025, 45(2): 707-716. |
| [8] | 田勋, 邓国刚, 羊晓东. 钴催化C(sp2)—H活化构建苯并含氮杂环骨架的研究进展[J]. 有机化学, 2025, 45(2): 655-667. |
| [9] | 邹瑜, 郭伟聪, 汪君. 不含手性取代基的平面手性环戊二烯基铑催化剂在不对称碳氢键活化中的应用[J]. 有机化学, 2025, 45(2): 466-476. |
| [10] | 袁晨晖, 焦雷. 手性配体在钯催化配位辅助对映选择性C(sp3)—H键官能团化反应中的应用[J]. 有机化学, 2025, 45(2): 602-619. |
| [11] | 徐晶, 张娟, 高文超, 孟凡会, 杨朋, 常宏宏. 疏水型催化剂在有机合成反应中的应用[J]. 有机化学, 2025, 45(1): 136-150. |
| [12] | 王耀伟, 王鹏, 史会兵, 张成富, 赵德明, 杨桂爱, 晏耀宗, 冯保林. 涉及人名反应的羰基化反应研究进展[J]. 有机化学, 2025, 45(1): 104-135. |
| [13] | 张腾飞, 常喆, 陈春霞, 彭进松. 过渡金属催化氮原子α位Csp3—H键官能团化反应研究进展[J]. 有机化学, 2025, 45(1): 168-188. |
| [14] | 慕晓楠, 管敏慧, 牛雨龙, 陈浩, 李传莹, 王磊. 光诱导重氮酸酯与偕二氟烯烃[1+2]环加成合成二氟环丙烷[J]. 有机化学, 2025, 45(1): 256-266. |
| [15] | 夏登鹏, 吴奇, 蔡志华, 杜广芬. 无催化条件下吲哚选择性硒化制备3-硒代吲哚[J]. 有机化学, 2025, 45(1): 349-357. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||