有机化学 ›› 2025, Vol. 45 ›› Issue (6): 1871-1904.DOI: 10.6023/cjoc202408035 上一篇 下一篇
综述与进展
马海宸a,†, 周钧岍a,†, 王嘉利a,†, 王优a,*( ), 朱少林a,b,c,*(
), 朱少林a,b,c,*( )
)
收稿日期:2024-08-29
修回日期:2024-10-15
发布日期:2024-11-27
通讯作者:
王优, 朱少林
作者简介:†共同第一作者.
基金资助:
Haichen Maa,†, Junqian Zhoua,†, Jiali Wanga,†, You Wanga,*( ), Shaolin Zhua,b,c,*(
), Shaolin Zhua,b,c,*( )
)
Received:2024-08-29
Revised:2024-10-15
Published:2024-11-27
Contact:
You Wang, Shaolin Zhu
About author:†The authors contributed equally to this work.
Supported by:文章分享

作为分子编辑的重要工具, 过渡金属催化的迁移偶联反应利用简单易得的原料, 结合金属动态迁移和选择性偶联来实现远程惰性C—H键的选择性官能团化, 从而简化合成路线, 提高合成效率. 近年来, 随着镍的迁移模式、多样性官能团化和选择性控制策略的发展, 镍催化的迁移偶联化学得到了快速的发展. 综述了镍催化的迁移偶联化学最新研究进展, 按照反应类型分为两部分: (1)迭代的1,2-金属迁移模式, 包括烯烃迁移单官能团化和烯烃迁移双官能团化(重点讨论迁移硼碳双官能团化), 实现远程C(sp3)—H键官能团化; (2)空间的1,4-金属迁移模式, 实现远程C(sp2)—H键官能团化.
马海宸, 周钧岍, 王嘉利, 王优, 朱少林. 镍催化的迁移偶联反应研究进展[J]. 有机化学, 2025, 45(6): 1871-1904.
Haichen Ma, Junqian Zhou, Jiali Wang, You Wang, Shaolin Zhu. Recent Advances in Ni-Catalyzed Migratory Cross-Coupling Reactions[J]. Chinese Journal of Organic Chemistry, 2025, 45(6): 1871-1904.

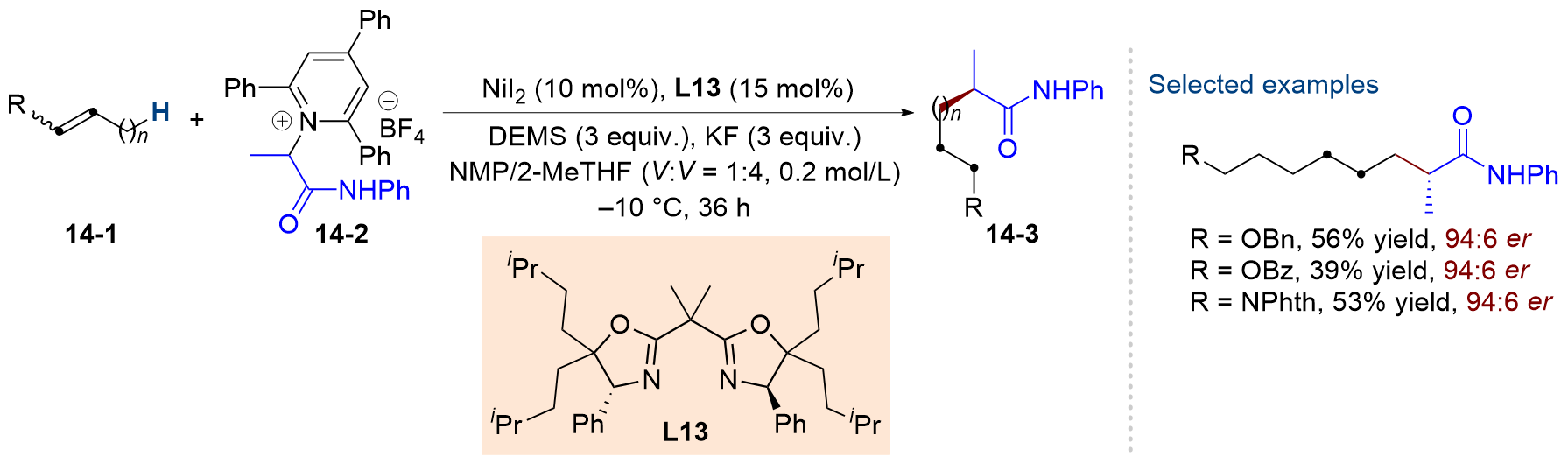






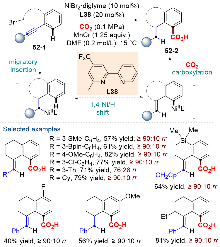

| [1] |
|
| [2] |
(a)
|
|
(b)
|
|
|
(c)
|
|
|
(d)
|
|
|
(e)
|
|
| [3] |
(a)
|
|
(b)
|
|
|
(c)
|
|
|
(d)
|
|
|
(e)
|
|
| [4] |
(a)
|
|
(b)
|
|
| [5] |
(a)
|
|
(b)
|
|
|
(c)
|
|
| [6] |
(a)
|
|
(b)
|
|
| [7] |
|
| [8] |
|
| [9] |
(a)
|
|
(b)
|
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
(a)
|
|
(b)
|
|
| [14] |
|
| [15] |
|
| [16] |
(a)
|
|
(b)
|
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
|
| [31] |
(a)
|
|
(b)
|
|
| [32] |
(a)
|
|
(b)
|
|
| [33] |
(a)
|
|
(b)
|
|
| [34] |
|
| [35] |
|
| [36] |
|
| [37] |
|
| [38] |
|
| [39] |
|
| [40] |
|
| [41] |
|
| [42] |
|
| [43] |
|
| [44] |
|
| [45] |
|
| [46] |
|
| [47] |
(a)
|
|
(b)
|
|
| [48] |
|
| [49] |
|
| [50] |
|
| [51] |
|
| [52] |
|
| [53] |
|
| [54] | |
| [55] |
|
| [56] |
|
| [57] |
|
| [58] |
|
| [59] |
|
| [60] |
|
| [61] |
|
| [62] |
|
| [63] |
|
| [1] | 高根伟, 李震, 李炎, 陆熹. 光/镍协同催化C(sp2)—C(sp3)键构建研究进展[J]. 有机化学, 2025, 45(6): 1905-1919. |
| [2] | 谢沈彤, 李文静, 刘钰, 陆熹, 师仁义. 镍催化多氟芳烃与烷基卤化物的还原烷基化反应[J]. 有机化学, 2025, 45(6): 2121-2127. |
| [3] | 刘俊杰, 赵红平, 胡媛媛, 汪恒昕, 袁伟明. 可见光诱导镍催化烯烃还原Heck反应[J]. 有机化学, 2025, 45(5): 1691-1697. |
| [4] | 郭仕勋, 王卫, 张永强. 三线态能量转移促进的自由基链式烯烃氢芳基化反应研究[J]. 有机化学, 2025, 45(5): 1716-1728. |
| [5] | 林恩泽, 李必杰. 基于碳氢键断裂的金属催化的内烯烃不对称氢芳基化进展[J]. 有机化学, 2025, 45(2): 546-558. |
| [6] | 厍远, 张书宇, 王乐. 内烯烃烯丙基选择性C—H键胺化反应研究进展[J]. 有机化学, 2025, 45(2): 531-545. |
| [7] | 慕晓楠, 管敏慧, 牛雨龙, 陈浩, 李传莹, 王磊. 光诱导重氮酸酯与偕二氟烯烃[1+2]环加成合成二氟环丙烷[J]. 有机化学, 2025, 45(1): 256-266. |
| [8] | 杜佳言, 刘俊涛, 刘桂霞, 黄正. 钴催化末端烯烃区域和立体选择性异构合成反式-2-烯烃[J]. 有机化学, 2024, 44(9): 2889-2897. |
| [9] | 吕雷阳, 罗亚妮, 李志平. 基于烯烃和炔烃的加成反应合成有机锗化合物的研究进展[J]. 有机化学, 2024, 44(7): 2092-2109. |
| [10] | 邢运新, 闫登鸿, 温顺, 卜洁, 沈坤. 镍催化1,6-烯炔与芳基卤化物的反式还原芳基化环化[J]. 有机化学, 2024, 44(6): 1938-1948. |
| [11] | 孙超, 周泉, 李传莹, 王磊. 苯并唑-氮杂环卡宾钯配合物的合成表征及应用[J]. 有机化学, 2024, 44(6): 1957-1966. |
| [12] | 陆玲依, 邱晓东. 自由基形式烯烃双烷基化反应研究进展[J]. 有机化学, 2024, 44(6): 1701-1718. |
| [13] | 于蕾, 盛康, 李亭, 唐从辉. 多相铁催化环状醚C(sp3)—H键活化的芳基烯烃氧烷基化[J]. 有机化学, 2024, 44(6): 1978-1986. |
| [14] | 史会兵, 王耀伟, 王鹏, 赵德明, 冯保林, 晏耀宗, 杨桂爱. 羰基化反应在大宗化学品以及精细化学品合成中的应用[J]. 有机化学, 2024, 44(6): 1811-1830. |
| [15] | 段东森, 马媛, 刘宇博, 程富, 朱道勇, 王少华. 可见光诱导的二氧化碳对活化烯烃的脱碳羧基化反应[J]. 有机化学, 2024, 44(5): 1675-1685. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||