有机化学 ›› 2025, Vol. 45 ›› Issue (12): 4271-4289.DOI: 10.6023/cjoc202505019 上一篇 下一篇
综述与进展
收稿日期:2025-05-16
修回日期:2025-07-01
发布日期:2025-08-18
通讯作者:
崔斌, 孙慧
基金资助:
Guo Zhong, Mengran Bai, Bin Cui*( ), Hui Sun*(
), Hui Sun*( )
)
Received:2025-05-16
Revised:2025-07-01
Published:2025-08-18
Contact:
Bin Cui, Hui Sun
Supported by:文章分享

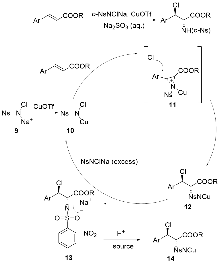
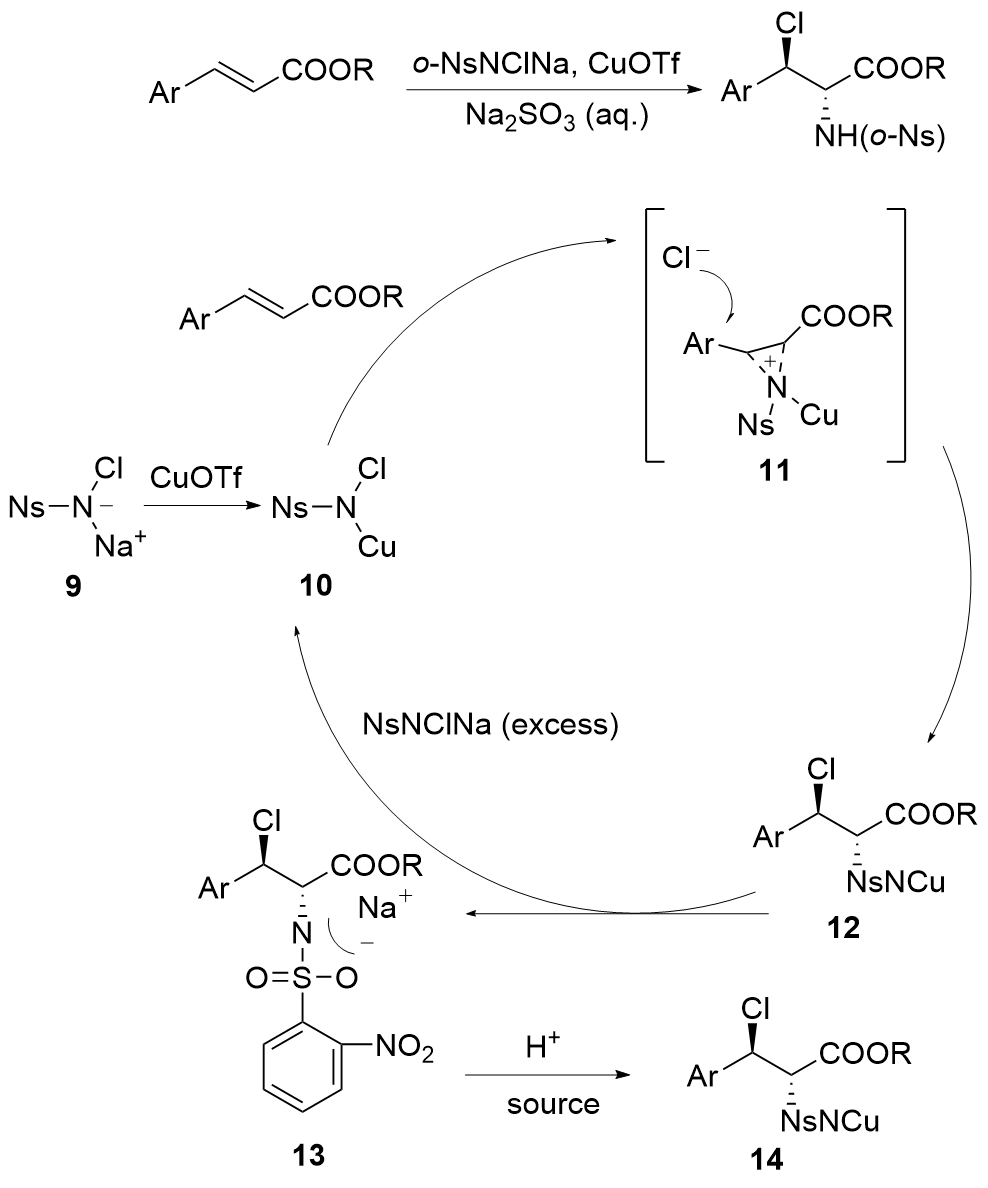
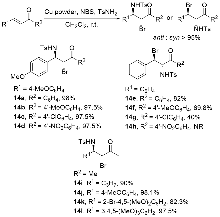
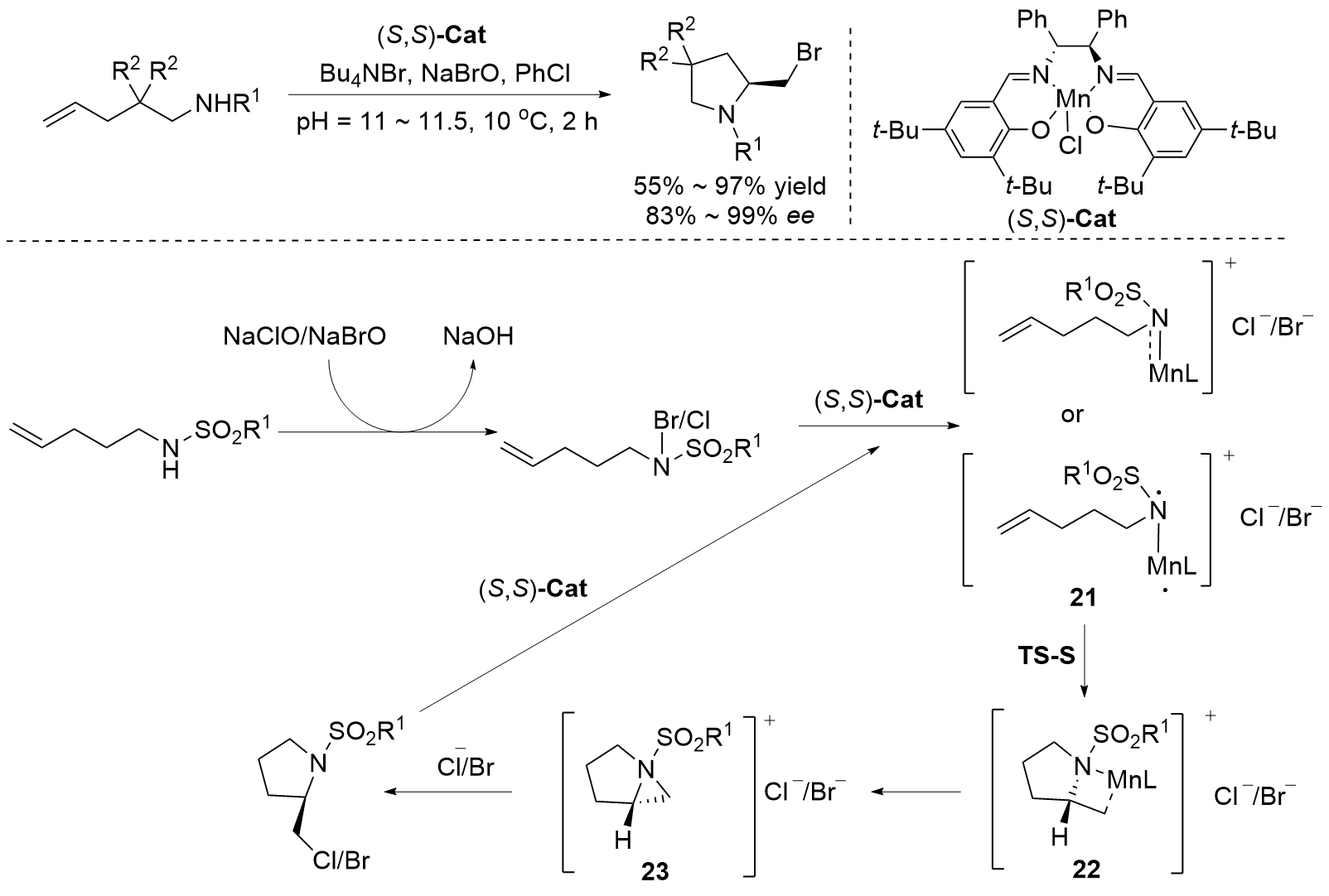
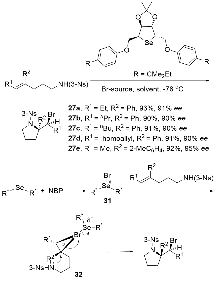
烯烃与胺基和卤素基的区域选择性和立体选择性相邻双官能化可以通过催化卤化方法有效地完成. 立体选择性卤胺化反应已成为将卤素官能团引入手性胺的重要方法, 在药物化学和有机合成中得到了广泛应用. 过去几十年来, 在催化系统和方法创新的推动下, 这一领域取得了重大进展. 使用手性催化剂对官能化和非官能化烯烃进行烯烃立体选择性卤胺化已成为一个突出的研究领域. 全面概述了过去几十年来立体选择性卤胺化领域取得的重大进展; 探讨了催化剂设计方面的创新, 这些创新促进了更高效、更具选择性的转化; 还分析了反应条件的优化, 这对提高这些反应的整体性能和适用性至关重要. 此外, 对立体选择性卤胺化的未来进行了展望.
钟国, 白萌冉, 崔斌, 孙慧. 烯烃立体选择性卤胺化的研究进展[J]. 有机化学, 2025, 45(12): 4271-4289.
Guo Zhong, Mengran Bai, Bin Cui, Hui Sun. Advances in Stereoselective Haloamination of Alkenes[J]. Chinese Journal of Organic Chemistry, 2025, 45(12): 4271-4289.



| [1] |
doi: 10.1002/ejoc.v2007:17 |
| [2] |
pmid: 23828735 |
| [3] |
pmid: 8609080 |
| [4] |
doi: 10.1021/np960602p |
| [5] |
|
| [6] |
doi: 10.1248/cpb.57.1142 |
| [7] |
doi: 10.1002/cber.v14:1 |
| [8] |
doi: 10.1002/cber.v17:2 |
| [9] |
doi: 10.1021/ja0304188 |
| [10] |
doi: 10.1002/1521-3773(20011119)40:22<>1.0.CO;2-D |
| [11] |
pmid: 12968342 |
| [12] |
doi: 10.1021/jo0200769 |
| [13] |
doi: 10.1021/jo030098a |
| [14] |
|
| [15] |
doi: 10.1021/ja00015a051 |
| [16] |
doi: 10.1139/v78-019 |
| [17] |
|
| [18] |
|
| [19] |
doi: 10.1055/s-2004-831203 |
| [20] |
doi: 10.1016/j.tetlet.2006.07.143 |
| [21] |
doi: 10.1021/ol050002u |
| [22] |
pmid: 11465449 |
| [23] |
|
| [24] |
doi: 10.1021/ol201895s |
| [25] |
doi: 10.1002/anie.v51.16 |
| [26] |
doi: 10.1016/j.tet.2013.05.022 |
| [27] |
doi: 10.1021/om049432z |
| [28] |
doi: 10.1021/ja9076588 |
| [29] |
doi: 10.1016/S0040-4020(01)90388-6 |
| [30] |
|
| [31] |
doi: 10.1248/cpb.15.1193 |
| [32] |
|
| [33] |
doi: 10.1021/jo01276a006 |
| [34] |
doi: 10.1055/s-2001-14910 |
| [35] |
doi: 10.1002/ejoc.v2004:14 |
| [36] |
|
| [37] |
doi: 10.1016/j.tet.2012.05.066 |
| [38] |
doi: 10.1021/ja00838a036 |
| [39] |
doi: 10.1016/j.tet.2016.09.038 |
| [40] |
doi: 10.1021/acs.orglett.1c02035 |
| [41] |
doi: 10.1021/acs.joc.2c01963 |
| [42] |
doi: 10.1021/ja00065a068 |
| [43] |
doi: 10.1021/ja00214a053 |
| [44] |
doi: 10.1021/cr00032a009 |
| [45] |
doi: 10.1021/ja00163a052 |
| [46] |
doi: 10.1021/ja981419g |
| [47] |
doi: 10.1021/ja00065a067 |
| [48] |
|
| [49] |
doi: 10.3891/acta.chem.scand.50-0649 |
| [50] |
doi: 10.1021/ol990059e |
| [51] |
pmid: 10930255 |
| [52] |
doi: 10.1016/S0040-4020(01)00228-9 |
| [53] |
doi: 10.1016/S0040-4020(01)00847-X |
| [54] |
doi: 10.1021/ol048045i |
| [55] |
doi: 10.1016/j.tetlet.2007.08.099 |
| [56] |
pmid: 11735517 |
| [57] |
doi: 10.1016/S0040-4039(01)02168-2 |
| [58] |
doi: 10.1016/j.tetlet.2003.11.049 |
| [59] |
doi: 10.1016/j.tetlet.2003.12.051 |
| [60] |
doi: 10.1002/ejoc.v2007:8 |
| [61] |
doi: 10.1021/ja110668c |
| [62] |
doi: 10.1039/c3cc44421j |
| [63] |
doi: 10.1021/acs.orglett.1c01642 |
| [64] |
doi: 10.1021/acs.orglett.3c00771 |
| [65] |
pmid: 12633091 |
| [66] |
doi: 10.1021/jo8023768 |
| [67] |
doi: 10.1016/j.tetlet.2013.02.113 |
| [68] |
doi: 10.1039/C7CC00470B |
| [69] |
|
| [70] |
|
| [71] |
doi: 10.1021/acs.joc.8b01951 |
| [72] |
doi: 10.1016/j.tetlet.2003.12.109 |
| [73] |
doi: 10.1021/acscatal.2c02223 |
| [74] |
doi: 10.1055/s-00000083 |
| [75] |
doi: 10.1021/ol101167z |
| [76] |
doi: 10.1021/jacs.8b02143 |
| [77] |
doi: 10.1002/anie.v61.32 |
| [78] |
doi: 10.3389/fchem.2020.00523 pmid: 32733847 |
| [79] |
doi: 10.1016/j.tet.2009.08.069 |
| [80] |
doi: 10.1021/ja201627h |
| [81] |
doi: 10.1039/C4CC05772D |
| [82] |
doi: 10.1039/c2ob25327e pmid: 22437158 |
| [83] |
doi: 10.1021/ja311202e |
| [84] |
|
| [85] |
|
| [86] |
doi: 10.3389/fchem.2021.742399 |
| [87] |
doi: 10.1002/chem.v20.41 |
| [88] |
doi: 10.1021/acscatal.8b03708 pmid: 31131150 |
| [89] |
doi: 10.1021/acs.orglett.0c03158 |
| [90] |
doi: 10.1021/acs.oprd.4c00489 |
| [1] | 徐程, 彭涛, 周梦圆, 刘明琳, 黄晓庆, 王永凤, 刘郑, 殷国栋. 室温下PPh3催化环丙烯酮与溴代烷烃的开环加成反应合成α,β-二取代丙烯酸酯[J]. 有机化学, 2026, 46(1): 279-288. |
| [2] | 高山, 解鑫, 刘元红. 镍/可见光协同催化的溴代芳烃与醇的C—O键偶联反应[J]. 有机化学, 2026, 46(1): 266-278. |
| [3] | 方霄龙, 王俊, 徐舒婷, 李浩, 胡坤, 陈宜瑜, Yiyu. 膦胺铼配合物催化酮加氢制醇性能研究[J]. 有机化学, 2026, 46(1): 241-249. |
| [4] | 任钶, 张光露, 牛轶凡, 王晓萌, 陈灿玉, 蒋敏. 光催化合成含氮杂芳环非天然氨基酸[J]. 有机化学, 2026, 46(1): 215-224. |
| [5] | 梁媛, 桂超, 王雯雯, 褚雪强, 徐浩, 沈志良. 镍催化的芳基全氟丁基磺酸酯通过碳-氧键官能化的硼化反应高效合成芳基硼酸酯[J]. 有机化学, 2026, 46(1): 207-214. |
| [6] | 谭紫云, 杨新, 龚绍峰, 杨慧翎, 谢永燕, 张静雅, 冯浙泰, 李文艺, 肖新生. 非均相铜催化的叔胺与炔烃需氧氧化交叉偶联反应[J]. 有机化学, 2026, 46(1): 156-166. |
| [7] | 王文龙, 温家旭, 陈飞, 薄春博, 李敏, 刘宁, 杜智宏. 铜催化的3-羟基-2-萘甲酸酯的不对称氧化偶联反应:氨基酸类配体的设计与优化[J]. 有机化学, 2026, 46(1): 167-180. |
| [8] | 刘佳乐, 唐裕才, 印丘梅, 黄嘉明, 邓世强, 夏伟铭, 蒋洁, 王祖利. 银催化(2,2-二氟-2)-苯硫基乙酸和2-芳基-N-丙烯酰胺吲哚的串联脱羧环化反应[J]. 有机化学, 2026, 46(1): 118-127. |
| [9] | 韩天娇, 师迁迁, 符运栋, 梅光建. 三酮烯烃与硝酮的反常区域选择性[3+2]环加成反应研究[J]. 有机化学, 2026, 46(1): 146-155. |
| [10] | 徐梦雅, 李一凡, 王越, 韩瑞萍, 李二庆. 银/Ganphos催化甲亚胺叶立德的对映选择性[3+2]环加成反应: 构筑螺旋环骨架[J]. 有机化学, 2025, 45(9): 3458-3468. |
| [11] | 杨威, 王兵兵, 徐小惠, 孙钟, 唐翠曼, 刘佳琦. 仿生材料聚多巴胺在有机转化中的应用进展[J]. 有机化学, 2025, 45(9): 3314-3325. |
| [12] | 祝辉, 吴鹏, 钟晨鸣, 李舒铭, 林钢, 刘雪粉, 罗书平. 供体-受体-供体(D-A-D)型芳香酮设计合成与光催化C(sp3)—H偶联反应性能研究[J]. 有机化学, 2025, 45(9): 3441-3449. |
| [13] | 贾晨旭, 安聪好, 黄军. 铜催化C—O偶联制备间苯氧基苯甲醛[J]. 有机化学, 2025, 45(9): 3412-3419. |
| [14] | 于志起, 陈灿源, 韩佳怡, 周建湖, 王林轩, 岳美娥, 贾肖飞. 多功能多孔有机聚合物负载钯纳米催化剂在炔烃氢羧基化中的应用[J]. 有机化学, 2025, 45(9): 3450-3457. |
| [15] | 赵倩倩, 魏培垚, 刘孙典, 张博鑫, 梁承远. 钯催化串联反应在含季碳中心的复杂天然产物全合成中的应用[J]. 有机化学, 2025, 45(9): 3289-3300. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||