有机化学 ›› 2025, Vol. 45 ›› Issue (9): 3441-3449.DOI: 10.6023/cjoc202501013 上一篇 下一篇
研究论文
祝辉a, 吴鹏a, 钟晨鸣a, 李舒铭a, 林钢a, 刘雪粉b, 罗书平a,*( )
)
收稿日期:2025-01-15
修回日期:2025-04-11
发布日期:2025-05-15
基金资助:
Hui Zhua, Peng Wua, Chenming Zhonga, Shuming Lia, Gang Lina, Xuefen Liub, Shuping Luoa,*( )
)
Received:2025-01-15
Revised:2025-04-11
Published:2025-05-15
Contact:
E-mail: Supported by:文章分享

设计合成了一系列以二酰基四氟苯为电子受体中心, 两侧连接多取代芳基为电子供体基团的供体-受体-供体(D-A-D)型芳香酮光敏剂(PC3~PC8), 并成功应用于甲基芳烃与芳基溴化物的C(sp3)—H偶联反应的光催化体系. 研究结果表明, 以间位二酰基四氟苯为电子受体中心, 以均三甲苯为供体基团的PC8表现出优异光催化性能, 其活性相比传统二苯甲酮提升460倍. 更值得注意的是, 成功活化了固态甲基芳烃类底物, 突破了甲基芳烃作反应溶剂的关键限制, 显著拓展了底物适用性范围. 理论计算表明, 光敏剂PC8的D-A-D结构能有效分离最高占据分子轨道(HOMO)与最低未占据分子轨道(LUMO)的电子云分布, 从而降低单重态-三重态能隙(ΔES-T), PC8的ΔES-T值(1.48 eV)明显低于二苯甲酮(1.84 eV), 有利于系间窜越(ISC)过程. 吸收光谱表明PC8在310 nm有特征吸收峰, 稳态荧光分析进一步证实, PC8的荧光量子效率为二苯甲酮的5倍, 上述光谱表征结果表明, PC8具有较好的光物理性能, 有效地促进了光催化循环的量子效率提升.
祝辉, 吴鹏, 钟晨鸣, 李舒铭, 林钢, 刘雪粉, 罗书平. 供体-受体-供体(D-A-D)型芳香酮设计合成与光催化C(sp3)—H偶联反应性能研究[J]. 有机化学, 2025, 45(9): 3441-3449.
Hui Zhu, Peng Wu, Chenming Zhong, Shuming Li, Gang Lin, Xuefen Liu, Shuping Luo. Design and Synthesis of Donor-Acceptor-Donor (D-A-D) Type Aromatic Ketones for Photocatalytic C(sp3)—H Coupling Reactions[J]. Chinese Journal of Organic Chemistry, 2025, 45(9): 3441-3449.
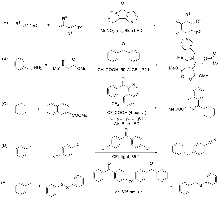

| Entry | Photocatalyst (mol%) | Solvent | Base | Time/h | Yieldb/% | |
|---|---|---|---|---|---|---|
| 1 | PC1 (20) | Toluene | NaHCO3 | 18 | 30 | |
| 2c | — | Toluene | NaHCO3 | 18 | None | |
| 3 | PC2 (20) | Toluene | NaHCO3 | 18 | 92 | |
| 4 | PC3 (20) | Toluene | NaHCO3 | 18 | 93 | |
| 5 | PC4 (20) | Toluene | NaHCO3 | 18 | 33 | |
| 6 | PC5 (20) | Toluene | NaHCO3 | 18 | 79 | |
| 7 | PC6 (20) | Toluene | NaHCO3 | 18 | 57 | |
| 8 | PC7 (20) | Toluene | NaHCO3 | 18 | 79 | |
| 9 | PC8 (20) | Toluene | NaHCO3 | 18 | 99 | |
| 10 | PC8 (5) | Toluene | NaHCO3 | 18 | 99 | |
| 11d | PC8 (5) | Toluene | NaHCO3 | 18 | None | |
| 12e | PC8 (5) | Toluene | NaHCO3 | 18 | None | |
| 13f | PC8 (5) | Toluene | NaHCO3 | 18 | None | |
| 14 | PC8 (5) | Toluene | NaHCO3 | 10 | 99 | |
| 15 | PC8 (5) | Toluene | K2HPO4 | 10 | 84 | |
| 16 | PC8 (5) | Toluene | CH3COONa | 10 | 23 | |
| 17 | PC8 (5) | Toluene | K2CO3 | 10 | 33 | |
| 18 | PC8 (5) | Toluene | Na2CO3 | 10 | 56 | |
| 19 | PC8 (5) | Toluene | NaHCO3 | 0.5 | 96 | |
| 20 | PC8 (5) | DMSO (0.1 mol/L) | NaHCO3 | 18 | None | |
| 21 | PC8 (5) | Hexane (0.1 mol/L) | NaHCO3 | 18 | Trace | |
| 22 | PC8 (5) | EtOAc (0.1 mol/L) | NaHCO3 | 18 | 7 | |
| 23 | PC8 (5) | MeCN (0.1 mol/L) | NaHCO3 | 18 | 32 | |
| 24 | PC8 (5) | MeCN (0.2 mol/L) | NaHCO3 | 18 | 55 | |
| 25 | PC8 (5) | MeCN (0.4 mol/L) | NaHCO3 | 18 | 99 | |
| 26 | PC8 (5) | MeCN (0.4 mol/L) | NaHCO3 | 0.5 | 87 | |
| Entry | Photocatalyst (mol%) | Solvent | Base | Time/h | Yieldb/% | |
|---|---|---|---|---|---|---|
| 1 | PC1 (20) | Toluene | NaHCO3 | 18 | 30 | |
| 2c | — | Toluene | NaHCO3 | 18 | None | |
| 3 | PC2 (20) | Toluene | NaHCO3 | 18 | 92 | |
| 4 | PC3 (20) | Toluene | NaHCO3 | 18 | 93 | |
| 5 | PC4 (20) | Toluene | NaHCO3 | 18 | 33 | |
| 6 | PC5 (20) | Toluene | NaHCO3 | 18 | 79 | |
| 7 | PC6 (20) | Toluene | NaHCO3 | 18 | 57 | |
| 8 | PC7 (20) | Toluene | NaHCO3 | 18 | 79 | |
| 9 | PC8 (20) | Toluene | NaHCO3 | 18 | 99 | |
| 10 | PC8 (5) | Toluene | NaHCO3 | 18 | 99 | |
| 11d | PC8 (5) | Toluene | NaHCO3 | 18 | None | |
| 12e | PC8 (5) | Toluene | NaHCO3 | 18 | None | |
| 13f | PC8 (5) | Toluene | NaHCO3 | 18 | None | |
| 14 | PC8 (5) | Toluene | NaHCO3 | 10 | 99 | |
| 15 | PC8 (5) | Toluene | K2HPO4 | 10 | 84 | |
| 16 | PC8 (5) | Toluene | CH3COONa | 10 | 23 | |
| 17 | PC8 (5) | Toluene | K2CO3 | 10 | 33 | |
| 18 | PC8 (5) | Toluene | Na2CO3 | 10 | 56 | |
| 19 | PC8 (5) | Toluene | NaHCO3 | 0.5 | 96 | |
| 20 | PC8 (5) | DMSO (0.1 mol/L) | NaHCO3 | 18 | None | |
| 21 | PC8 (5) | Hexane (0.1 mol/L) | NaHCO3 | 18 | Trace | |
| 22 | PC8 (5) | EtOAc (0.1 mol/L) | NaHCO3 | 18 | 7 | |
| 23 | PC8 (5) | MeCN (0.1 mol/L) | NaHCO3 | 18 | 32 | |
| 24 | PC8 (5) | MeCN (0.2 mol/L) | NaHCO3 | 18 | 55 | |
| 25 | PC8 (5) | MeCN (0.4 mol/L) | NaHCO3 | 18 | 99 | |
| 26 | PC8 (5) | MeCN (0.4 mol/L) | NaHCO3 | 0.5 | 87 | |


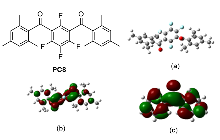

| Entry | PC | ELUMO/eV | EHOMO/eV | ΔE/eV | ΔES-T/eV |
|---|---|---|---|---|---|
| 1 | PC1 | -2.08 | -6.86 | 4.78 | 1.84 |
| 2 | PC2 | -2.76 | -7.76 | 5.00 | 2.06 |
| 3 | PC3 | -2.64 | -7.57 | 4.94 | 2.00 |
| 4 | PC8 | -2.50 | -6.92 | 4.42 | 1.48 |
| Entry | PC | ELUMO/eV | EHOMO/eV | ΔE/eV | ΔES-T/eV |
|---|---|---|---|---|---|
| 1 | PC1 | -2.08 | -6.86 | 4.78 | 1.84 |
| 2 | PC2 | -2.76 | -7.76 | 5.00 | 2.06 |
| 3 | PC3 | -2.64 | -7.57 | 4.94 | 2.00 |
| 4 | PC8 | -2.50 | -6.92 | 4.42 | 1.48 |

| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
doi: 10.1039/d2sc04990b pmid: 36382292 |
| [22] |
|
| [23] |
|
| [24] |
doi: 10.7536/PC230306 |
| [25] |
doi: 10.1021/ja307861n pmid: 23025482 |
| [26] |
doi: 10.1021/acscatal.9b01284 |
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
|
| [31] |
|
| [32] |
|
| [33] |
|
| [34] |
|
| [35] |
|
| [36] |
|
| [37] |
|
| [38] |
doi: 10.1002/anie.201802656 pmid: 29566297 |
| [39] |
|
| [1] | 贾晨旭, 安聪好, 黄军. 铜催化C—O偶联制备间苯氧基苯甲醛[J]. 有机化学, 2025, 45(9): 3412-3419. |
| [2] | 许文, 罗美明, 曾小明. 铬催化三氟甲基烯烃的还原交叉偶联[J]. 有机化学, 2025, 45(9): 3401-3411. |
| [3] | 王楠, 汤文军. 大位阻膦配体在钯催化芳基烷基交叉偶联反应中的应用★[J]. 有机化学, 2025, 45(9): 3351-3360. |
| [4] | 解人杰, 谢复开, 孙然, 王欣, 王钰佳, 李蕾, 王贺. 电子供体-受体复合物(EDA)介导N-芳基丙烯酰胺与芳基硫鎓盐的自由基环化反应[J]. 有机化学, 2025, 45(8): 2913-2922. |
| [5] | 李金霞, 邓远程, 李嘉瑜, 郭益豪, 王广凤, 段阿冰, 瞿双林. 钯催化硅环开环/交叉偶联反应机理研究[J]. 有机化学, 2025, 45(8): 2938-2944. |
| [6] | 史茜, 李忠玉, 李晗. 杂环金属铱配合物光敏剂的研究进展[J]. 有机化学, 2025, 45(7): 2389-2405. |
| [7] | 王静, 陈宇讯, 姜静静. 新型茚并芴-5,7-二酮衍生物的合成与性质研究[J]. 有机化学, 2025, 45(7): 2444-2450. |
| [8] | 王超, 陈洪平, 米明众, 李澳文, 齐燕, 刘永军. 锰催化的有机合成偶联反应进展[J]. 有机化学, 2025, 45(7): 2326-2334. |
| [9] | 谭永波, 舒洪波, 黄华文. 光诱导N-芳基丙烯酰胺参与的吲哚酮合成研究进展[J]. 有机化学, 2025, 45(6): 2086-2108. |
| [10] | 杜一鸣, 贾均松, 李玉龙, 舒伟. 手性α-芳基酮的催化合成研究进展[J]. 有机化学, 2025, 45(6): 1838-1870. |
| [11] | 苏雷, 杨熙, 闫捷, 蒋元力, 陈丽娟, 郑庆舒, 刘家旺. 不对称羰基化偶联反应研究进展[J]. 有机化学, 2025, 45(6): 2007-2047. |
| [12] | 高根伟, 李震, 李炎, 陆熹. 光/镍协同催化C(sp2)—C(sp3)键构建研究进展[J]. 有机化学, 2025, 45(6): 1905-1919. |
| [13] | 李顺曦, 游力栩, 李玉龙, 舒伟. 光介导氨及其等价体参与的碳氮成键反应研究进展[J]. 有机化学, 2025, 45(5): 1460-1477. |
| [14] | 周思成, 刘运奎. P/N-杂配铜(I)光催化剂介导的可见光催化反应进展[J]. 有机化学, 2025, 45(5): 1644-1668. |
| [15] | 陈雨佳, 刘志林, 陈凯, 向皞月, 阳华. 无金属、光催化氧化苄基C—H键以获得羰基官能团[J]. 有机化学, 2025, 45(5): 1755-1762. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||