有机化学 ›› 2026, Vol. 46 ›› Issue (1): 233-240.DOI: 10.6023/cjoc202506003 上一篇 下一篇
研究论文
刘涛a, 周永博a, 郎同庆b, 牛海波b, 陈飞a, 杜智宏a, 薄春博a, 李敏a,*( ), 刘宁a,*(
), 刘宁a,*( )
)
收稿日期:2025-06-02
修回日期:2025-07-28
发布日期:2025-09-03
基金资助:
Tao Liua, Yongbo Zhoua, Tongqing Langb, Haibo Niub, Fei Chena, Zhihong Dua, Chunbo Boa, Min Lia,*( ), Ning Liua,*(
), Ning Liua,*( )
)
Received:2025-06-02
Revised:2025-07-28
Published:2025-09-03
Contact:
* E-mail: ningliu@shzu.edu.cn;
limin@shzu.edu.cn
Supported by:文章分享
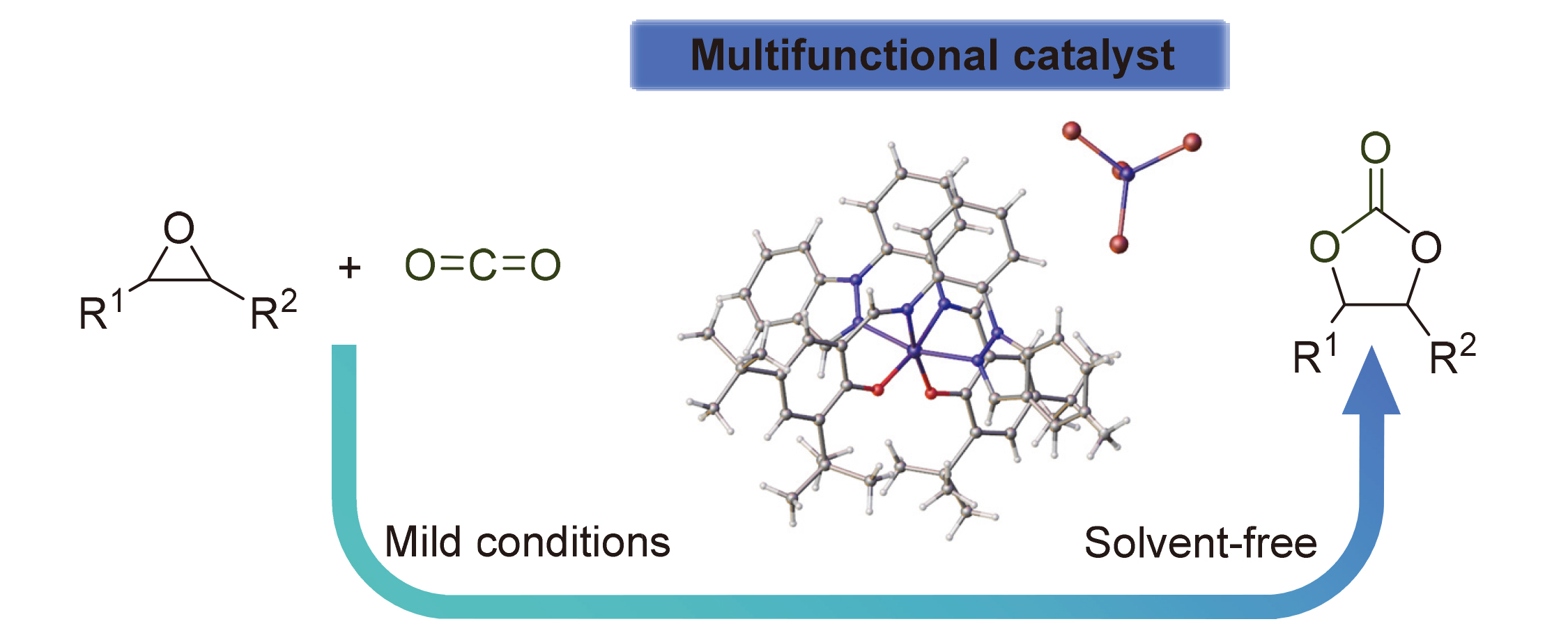
为实现二氧化碳(CO₂)的高值化利用, 合成了两个席夫碱型Fe(III)配合物, 探究其在CO₂与环氧化物环加成反应中制备环状碳酸酯的催化性能. 结果表明, 双((((2-(1H-吡唑-1-基)苯基)亚氨基)甲基)-4,6-二叔丁基苯酚)合铁(III)•四溴化铁(III) (Cat 2)在80 ℃、0.50 MPa CO2压力下, 无需溶剂和助催化剂, 对多种环氧化物(包括末端、芳香族及高位阻环氧化物)表现出高催化活性和底物普适性, 收率可达89%~94%. 通过X射线光电子能谱(XPS)和高分辨率质谱(HRMS)分析, 明确了Fe(III)价态及活性中间体, 并提出了催化机理: Fe(III)中心作为路易斯酸活化环氧化物, 阴离子四溴化铁中解离出来的溴离子充当亲核试剂, 促进环氧化物开环, 二者协同作用促进反应. 该Fe催化体系为CO₂转化提供了一种催化剂设计思路.
刘涛, 周永博, 郎同庆, 牛海波, 陈飞, 杜智宏, 薄春博, 李敏, 刘宁. 席夫碱型Fe(III)配合物催化二氧化碳和环氧化物制备环状碳酸酯[J]. 有机化学, 2026, 46(1): 233-240.
Tao Liu, Yongbo Zhou, Tongqing Lang, Haibo Niu, Fei Chen, Zhihong Du, Chunbo Bo, Min Li, Ning Liu. Schiff Base Type Fe(III) Complexes Catalyzed Carbon Dioxide and Epoxides for Preparing Cyclic Carbonates[J]. Chinese Journal of Organic Chemistry, 2026, 46(1): 233-240.
| Entry | Catalyst | Catalyst/mol% | Temp./℃ | Yieldb/% |
|---|---|---|---|---|
| 1 | Cat 1 | 1.00 | 30 | 50 |
| 2 | Cat 2 | 1.00 | 30 | 60 |
| 3 | Cat 2 | 1.00 | 80 | 94 |
| 4 | Cat 2 | 0.75 | 80 | 90 |
| 5 | Cat 2 | 0.50 | 80 | 89 |
| 6 | Cat 2 | 0.30 | 80 | 60 |
| Entry | Catalyst | Catalyst/mol% | Temp./℃ | Yieldb/% |
|---|---|---|---|---|
| 1 | Cat 1 | 1.00 | 30 | 50 |
| 2 | Cat 2 | 1.00 | 30 | 60 |
| 3 | Cat 2 | 1.00 | 80 | 94 |
| 4 | Cat 2 | 0.75 | 80 | 90 |
| 5 | Cat 2 | 0.50 | 80 | 89 |
| 6 | Cat 2 | 0.30 | 80 | 60 |
| Entry | Temp./℃ | p(CO2)/MPa | Time/h | Yieldb/% |
|---|---|---|---|---|
| 1 | 30 | 1.00 | 18 | 60 |
| 2 | 60 | 1.00 | 18 | 85 |
| 3 | 80 | 1.00 | 18 | 94 |
| 4 | 100 | 1.00 | 18 | 96 |
| 5 | 80 | 0.30 | 18 | 75 |
| 6 | 80 | 0.50 | 18 | 90 |
| 7 | 80 | 0.75 | 18 | 92 |
| 8 | 80 | 1.00 | 18 | 94 |
| 9 | 80 | 0.50 | 4 | 82 |
| 10 | 80 | 0.50 | 8 | 89 (90) c |
| 11 | 80 | 0.50 | 12 | 90 |
| 12 | 80 | 0.50 | 18 | 92 |
| Entry | Temp./℃ | p(CO2)/MPa | Time/h | Yieldb/% |
|---|---|---|---|---|
| 1 | 30 | 1.00 | 18 | 60 |
| 2 | 60 | 1.00 | 18 | 85 |
| 3 | 80 | 1.00 | 18 | 94 |
| 4 | 100 | 1.00 | 18 | 96 |
| 5 | 80 | 0.30 | 18 | 75 |
| 6 | 80 | 0.50 | 18 | 90 |
| 7 | 80 | 0.75 | 18 | 92 |
| 8 | 80 | 1.00 | 18 | 94 |
| 9 | 80 | 0.50 | 4 | 82 |
| 10 | 80 | 0.50 | 8 | 89 (90) c |
| 11 | 80 | 0.50 | 12 | 90 |
| 12 | 80 | 0.50 | 18 | 92 |
| [1] |
doi: 10.1002/anie.v55.26 |
| [2] |
doi: 10.1016/j.ccr.2010.12.002 |
| [3] |
doi: 10.1021/cr4002758 |
| [4] |
|
| [5] |
doi: 10.1002/cssc.v7.7 |
| [6] |
doi: 10.1039/C4GC01719F |
| [7] |
doi: 10.1039/D0GC01870H |
| [8] |
doi: 10.1039/D0GC03465G |
| [9] |
doi: 10.1021/acssuschemeng.8b00102 |
| [10] |
doi: 10.1021/acs.organomet.8b00795 |
| [11] |
doi: 10.1002/cctc.v10.4 |
| [12] |
doi: 10.1016/j.molcata.2016.04.018 |
| [13] |
|
| [14] |
doi: 10.1039/D0DT03887C |
| [15] |
doi: 10.1021/acs.inorgchem.4c03640 |
| [16] |
|
| [17] |
doi: 10.1039/C6GC00370B |
| [18] |
doi: 10.1021/acscatal.6b01386 |
| [19] |
doi: 10.1021/acs.inorgchem.9b00262 |
| [20] |
|
| [21] |
doi: 10.1002/cctc.201900745 |
| [22] |
|
|
(张湘南, 马宏方, 钱炜鑫, 张海涛, 应卫勇, 低碳化学与化工, 2025, 50, 20.)
|
|
| [23] |
doi: 10.6023/cjoc202206047 |
|
(刘桂杰, 付正强, 陈飞, 徐彩霞, 李敏, 刘宁, 有机化学, 2023, 43, 629.)
doi: 10.6023/cjoc202206047 |
|
| [24] |
doi: 10.6023/cjoc202405037 |
|
(李建文, 王涛, 陶晟, 陈飞, 李敏, 刘宁, 有机化学, 2024, 44, 3213.)
doi: 10.6023/cjoc202405037 |
|
| [25] |
doi: 10.3390/nano12193462 |
| [26] |
doi: 10.1021/cr500425u |
| [27] |
doi: 10.1021/ic3008624 |
| [28] |
doi: 10.1039/C6CY00477F |
| [29] |
doi: 10.1021/acssuschemeng.7b01990 |
| [30] |
doi: 10.1002/cssc.201801065 pmid: 29897168 |
| [31] |
|
| [32] |
doi: 10.1016/j.apcatb.2020.119395 |
| [33] |
doi: 10.1021/acschemneuro.2c00728 |
| [34] |
doi: 10.1021/acsami.1c04624 |
| [35] |
doi: 10.1021/acs.inorgchem.4c00461 |
| [36] |
doi: 10.1021/acs.inorgchem.4c02452 |
| [37] |
doi: 10.1021/acs.joc.9b00997 |
| [38] |
doi: 10.3390/molecules20058395 |
| [39] |
doi: 10.1002/adsc.v354.2/3 |
| [40] |
doi: 10.1002/adsc.v358.4 |
| [41] |
doi: 10.1021/acs.inorgchem.1c01542 |
| [42] |
doi: 10.1021/acscatal.3c02449 |
| [43] |
doi: 10.1021/acs.organomet.0c00525 |
| [44] |
doi: 10.1039/C6GC00671J |
| [1] | 徐程, 彭涛, 周梦圆, 刘明琳, 黄晓庆, 王永凤, 刘郑, 殷国栋. 室温下PPh3催化环丙烯酮与溴代烷烃的开环加成反应合成α,β-二取代丙烯酸酯[J]. 有机化学, 2026, 46(1): 279-288. |
| [2] | 陈艺林, 岳佳怡, 林依鸣, 刘兵妮, 戴闻, 朱红平. 硅杂环丙烯的合成和表征[J]. 有机化学, 2026, 46(1): 128-134. |
| [3] | 徐梦雅, 李一凡, 王越, 韩瑞萍, 李二庆. 银/Ganphos催化甲亚胺叶立德的对映选择性[3+2]环加成反应: 构筑螺旋环骨架[J]. 有机化学, 2025, 45(9): 3458-3468. |
| [4] | 张森, 江凌云, 辛雨, 黄年玉, 王能中. 有机碱促进的芳基乙二醛一水合物与烯丙基鏻盐的[2+3]环加成反应: 高效合成环戊烯酮类化合物[J]. 有机化学, 2025, 45(9): 3420-3428. |
| [5] | 冯保林, 王鹏, 赵德明, 杨桂爱, 晏耀宗, 史会兵, 王耀伟. CO替代物参与羰基化反应研究进展[J]. 有机化学, 2025, 45(8): 2773-2795. |
| [6] | 马惠敏, 赵宇含, 黄业伟, 孔令斌. 水介质中氰基环氧化物的化学选择性绿色合成研究[J]. 有机化学, 2025, 45(8): 2867-2875. |
| [7] | 刘尊棋, Jahangir Khan, Muhammad Akram, Yasir Mumtaz. 异硫氰酸盐合成与应用的最新进展[J]. 有机化学, 2025, 45(7): 2350-2366. |
| [8] | 陈刚, 陈东, 聂广杰, 李林轩, 姚辉, 王能中, 黄年玉. 有机膦催化下橙酮衍生的氮杂二烯与氨基巴豆酸酯的[2+3]环加成反应[J]. 有机化学, 2025, 45(6): 2139-2148. |
| [9] | 王涛, 陶晟, 陈飞, 杜智宏, 薄春博, 李敏, 刘宁. 双功能钼配合物催化二氧化碳和环氧化物制备环状碳酸酯[J]. 有机化学, 2025, 45(12): 4354-4361. |
| [10] | 张业飞, 唐勇, 周友运. 共轭二烯参与的[4+1]环加成反应研究进展[J]. 有机化学, 2025, 45(1): 1-21. |
| [11] | 金欢, 朱彦军, 李芳, 罗云飞. 新型含酯基官能团的茂基金属铑催化剂的合成及其在催化合成异喹啉反应中的应用[J]. 有机化学, 2024, 44(9): 2906-2914. |
| [12] | 蒋镓西, 刘全忠. 乙烯基重氮化合物非金属卡宾机制参与的反应[J]. 有机化学, 2024, 44(9): 2640-2657. |
| [13] | 段东森, 马媛, 刘宇博, 程富, 朱道勇, 王少华. 可见光诱导的二氧化碳对活化烯烃的脱碳羧基化反应[J]. 有机化学, 2024, 44(5): 1675-1685. |
| [14] | 姜晓琳, 王超洋, 武利园, 李跃辉. 含咔唑结构的小分子及聚合物催化二氧化碳转化研究进展[J]. 有机化学, 2024, 44(5): 1423-1444. |
| [15] | 夏坤, 张开发, Sher Wali Khan, 阿布力米提•阿布都卡德尔. 二氧化碳参与的三组分偶联反应进展[J]. 有机化学, 2024, 44(5): 1506-1525. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||