有机化学 ›› 2025, Vol. 45 ›› Issue (1): 1-21.DOI: 10.6023/cjoc202407043 上一篇 下一篇
综述与进展
收稿日期:2024-07-29
修回日期:2024-09-04
发布日期:2024-09-18
基金资助:
Yefei Zhanga,b, Yong Tangb,c( ), You-Yun Zhoub(
), You-Yun Zhoub( )
)
Received:2024-07-29
Revised:2024-09-04
Published:2024-09-18
Contact:
*E-mail: Supported by:文章分享

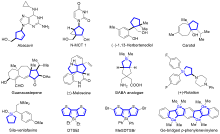
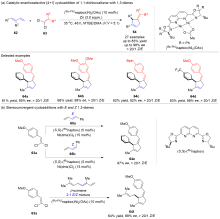
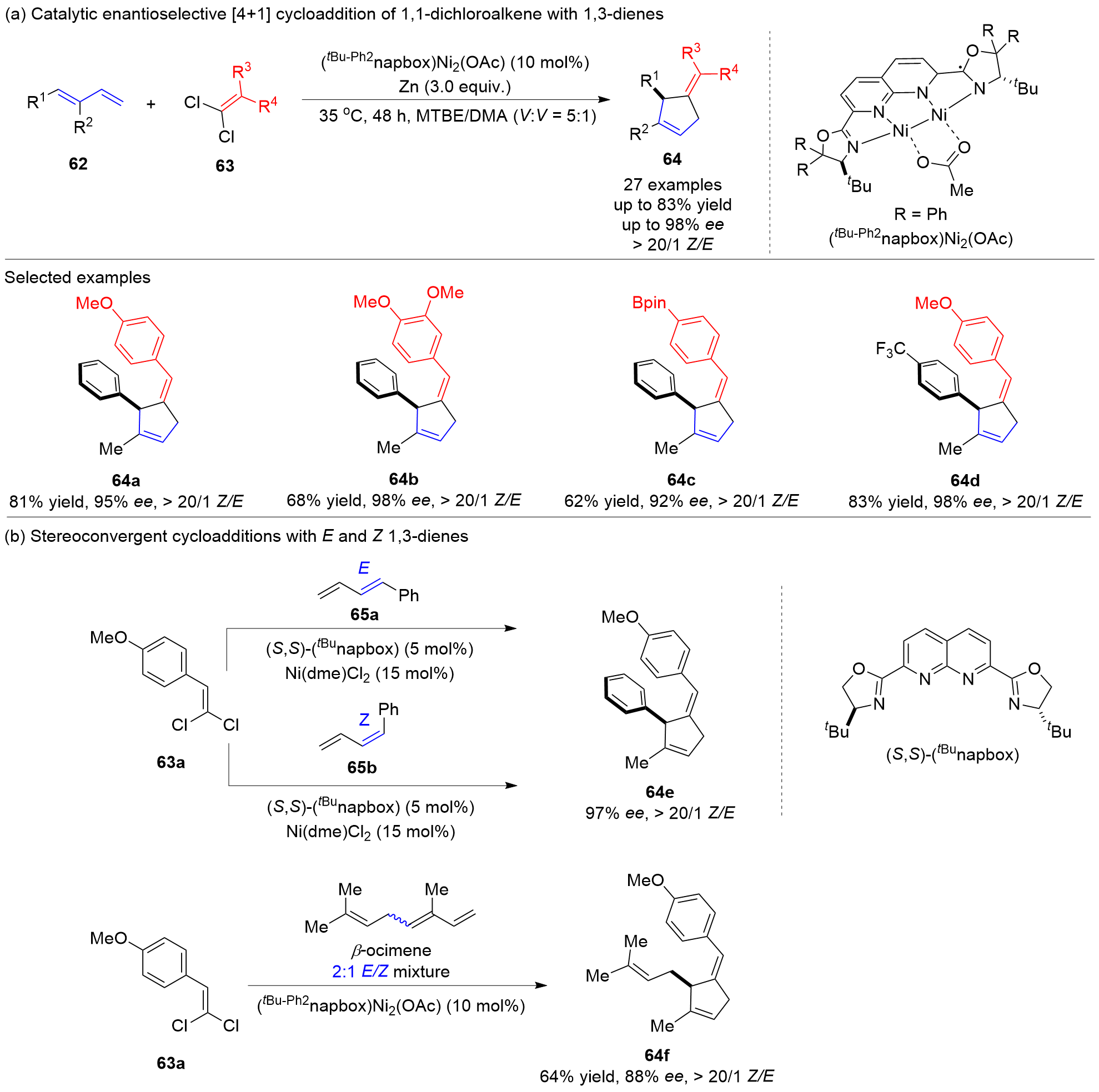
五元环是广泛存在于天然产物、药物和功能材料中的一类重要结构单元, 也是有机合成中常用的重要中间体和药物开发的优选骨架. 因此, 五元环化合物的高效合成一直是有机合成领域的重要研究方向之一. 廉价易得、结构多样的共轭二烯参与的[4+1]环加成反应是一种具有步骤经济性的直接构建五元碳环和杂环化合物的高效方法和策略, 备受有机合成界的青睐. 近年来, 随着过渡金属催化体系和“一原子”合成子前体的开发, 此类将简单易得原料转化成结构复杂和官能团多样化的五元环状分子的[4+1]环加成反应得到了较快的发展, 很多优秀的成果被报道出来. 详细介绍了共轭二烯与“一原子”合成子(一氧化碳、卡宾、硅宾以及氮宾)的[4+1]环加成反应的研究进展, 并按照合成子的类型进行了分类讨论, 最后总结了该领域存在的挑战, 展望了未来的研究方向.
张业飞, 唐勇, 周友运. 共轭二烯参与的[4+1]环加成反应研究进展[J]. 有机化学, 2025, 45(1): 1-21.
Yefei Zhang, Yong Tang, You-Yun Zhou. Progress in [4+1] Cycloadditions of Conjugated Dienes[J]. Chinese Journal of Organic Chemistry, 2025, 45(1): 1-21.

| [1] |
(a) Das, S.; Chandrasekhar, S.; Yadav, J. S.; Gree, R. Chem. Rev. 2007, 107, 3286.
|
|
(b) Kurteva, V. B.; Afonso, C. A. M. Chem. Rev. 2009, 109, 6809.
|
|
|
(c) Vitaku, E.; Smith, D. T.; Njardarson, J. T. J. Med. Chem. 2014, 57, 10257.
|
|
|
(d) Ni, H. Q.; Cooper, P.; Engle, K. M. Chem. Commun. 2021, 57, 7610.
|
|
|
(e) Marshall, C. M.; Federice, J. G.; Bell, C. N.; Cox, P. B.; Njar-darson, J. T. J. Med. Chem. 2024, 67, 11622.
|
|
| [2] |
(a) Ohshita, J. Macromol. Chem. Phys. 2009, 210, 1360.
|
|
(b) Dong, Z.; Albers, L.; Müller, T. Acc. Chem. Res. 2020, 53, 532.
|
|
| [3] |
(a) Padwa, A.; Pearson, W. H. Synthetic Applications of 1, 3-Dipolar Cycloaddition Chemistry toward Heterocycles and Natural Products, Wiley-Interscience, New York, 2002.
|
|
For reviews: (b) Gothelf, K. V.; Jørgensen, K. A. Chem. Rev. 1998, 98, 863.
|
|
|
(c) Coldham, I.; Hufton, R. Chem. Rev. 2005, 105, 2765.
|
|
|
(d) Pandey, G.; Banerjee, P.; Gadre, S. R. Chem. Rev. 2006, 106, 4484.
|
|
|
(e) Stanley, L. M.; Sibi, M. P. Chem. Rev. 2008, 108, 2887.
|
|
|
(f) Adrio, J.; Carretero, J. C. Chem. Commun. 2011, 47, 6784.
|
|
|
(g) Adrio, J.; Carretero, J. C. Chem. Commun. 2014, 50, 12434.
|
|
| [4] |
(a) Torres, R. R. The Pauson-Khand Reaction: Scope, Variations and Applications, Wiley-VCH, Weinheim, 2012.
|
|
For reviews: (b) Gibson, S. E.; Stevenazzi, A. Angew. Chem., Int. Ed. 2003, 42, 1800.
|
|
|
(c) Blanco-Urgoiti, J.; Anorbe, L.; Perez-Serrano, L.; Dominguez, G.; Perez-Castells, J. Chem. Soc. Rev. 2004, 33, 32.
|
|
|
(d) Shibata, T. Adv. Synth. Catal. 2006, 348, 2328.
|
|
|
(e) Lee, H. W.; Kwong, F. Y. Eur. J. Org. Chem. 2010, 789.
|
|
| [5] |
(a) Hudlicky, T.; Price, J. D. Chem. Rev. 1989, 89, 1467.
|
|
(b) Wong, H. N. C.; Hon, M. Y.; Tse, C. W.; Yip, Y. C.; Tanko, J.; Hudlicky, T. Chem. Rev. 1989, 89, 165.
|
|
|
(c) Baldwin, J. E. Chem. Rev. 2003, 103, 1197.
|
|
|
(d) Baldwin, J. E.; Leber, P. A. Org. Biomol. Chem. 2008, 6, 36.
|
|
|
(e) Hudlicky, T.; Reed, J. W. Angew. Chem., Int. Ed. 2010, 49, 4864.
|
|
| [6] |
(a) Lu, L. Q.; Zhang, J. J.; Li, F.; Cheng, Y.; An, J.; Chen, J. R.; Xiao, W. J. Angew. Chem., Int. Ed. 2010, 49, 4495.
|
|
(b) Wang, Q.; Qi, X. T.; Lu, L. Q.; Li, T. R.; Yuan, Z. G.; Zhang, K.; Li, B. J.; Lan, Y.; Xiao, W. J. Angew. Chem., Int. Ed. 2016, 55, 2840.
|
|
|
(c) Wang, Q.; Li, T. R.; Lu, L. Q.; Li, M. M.; Zhang, K.; Xiao, W. J. J. Am. Chem. Soc. 2016, 138, 8360.
|
|
|
(d) Liu, H.; He, G. C.; Zhao, C. Y.; Zhang, X. X.; Ji, D. W.; Hu, Y. C.; Chen, Q. A. Angew. Chem., Int. Ed. 2021, 60, 24284.
|
|
|
(e) Kong, H. H.; Zhu, C. J.; Deng, S.; Xu, G.; Zhao, R. N.; Yao, C. C.; Xiang, H. M.; Zhao, C. H.; Qi, T. T.; Xu, H. J. Am. Chem. Soc. 2022, 144, 21347.
|
|
|
(f) Qu, B. L.; Shi, B.; He, L.; Shi, J. W.; Xiao, W. J.; Lu, L. Q. Org. Chem. Front. 2023, 10, 3498.
|
|
|
For reviews: (g) Li, Y.; Feng, K. L.; Zhao, R. N.; Zhu, C. J.; Xu, H. Synlett 2024, 35, 1089.
|
|
| [7] |
(a) Murakami, M.; Itami, K.; Ito, Y. Angew. Chem., Int. Ed. Engl. 1996, 34, 2691.
|
|
(b) Murakami, M.; Itami, K.; Ito, Y. J. Am. Chem. Soc. 1997, 119, 2950.
|
|
|
(c) Murakami, M.; Itami, K.; Ito, Y. Organometallics 1999, 18, 1326.
|
|
|
(d) Murakami, M.; Itami, K.; Ito, Y. J. Am. Chem. Soc. 1999, 121, 4130.
|
|
|
(e) Chatani, N. In Ruthenium Catalysts and Fine Chemistry, Vol. 11, Eds.: Bruneau, C.; Dixneuf, P., Springer, Berlin Heidelberg, 2004.
|
|
|
(f) Kollar, L. Modern Carbonylation Methods, Wiley-VCH, Weinheim, 2008.
|
|
|
For a review: (g) Li, C. L.; Yu, Z. X. Chin. J. Org. Chem. 2024, 44, 1045 (in Chinese).
|
|
|
(李晨龙, 余志祥, 有机化学, 2024, 44, 1045.)
|
|
| [8] |
Frühauf, H. W. Chem. Rev. 1997, 97, 523.
|
| [9] |
Johnson, B. F. G.; Lewis, J.; Thompson, D. J. Tetrahedron Lett. 1974, 15, 3789.
|
| [10] |
(a) Franck-Neumann, M.; Vernier, J. M. Tetrahedron Lett. 1992, 33, 7361.
|
|
(b) Franck-Neumann, M.; Michelotti, E. L.; Simler, R.; Vernier, J. M. Tetrahedron Lett. 1992, 33, 7365.
|
|
| [11] |
Yang, Y. S.; Li, H. X.; Zhu, Y. T.; Zhang, Z. Y.; Yu, Z. X. J. Am. Chem. Soc. 2023, 145, 17087.
|
| [12] |
Chen, J. R.; Hu, X. Q.; Lu, L. Q.; Xiao, W. J. Chem. Rev. 2015, 115, 5301.
|
| [13] |
(a) Fujimoto, H.; Hoffmann, R. J. Phys. Chem. 1974, 78, 1167.
|
|
(b) Schoeller, W. W.; Yurtsever, E. J. Am. Chem. Soc. 1978, 100, 7548.
|
|
|
(c) Bauld, N. L.; Wirth, D. J. Comput. Chem. 1981, 2, 1.
|
|
|
(d) Moss, R. A.; Jones, M. Jr. In Reactive Intermediates, Vol. 2, Wiley, New York, 1981, Chapter 3.
|
|
|
(e) Schoeller, W. W.; Aktekin, N. J. Chem. Soc., Chem. Commun. 1982, 20.
|
|
|
(f) Evanseck, J. D.; Mareda, J.; Houk, K. N. J. Am. Chem. Soc. 1990, 112, 73.
|
|
| [14] |
Schnaubelt, J.; Marks, E.; Reibig, H. U. Chem. Ber. 1996, 129, 73.
|
| [15] |
(a) Salomon, R. G., Salomon, M. E.; Kachinsky, J. L. C. J. Am. Chem. Soc. 1977, 99, 1043.
|
|
(b) Doyle, M. P.; Van Leusen, D. J. Am. Chem. Soc. 1981, 103, 5917.
|
|
|
(c) Hudlicky, T.; Kutchan, T. M.; Naqvi, S. M. Org. React. 1985, 33, 247.
|
|
| [16] |
Hofmann, B.; Reibig, H. U. Chem. Ber. 1994, 127, 2315.
|
| [17] |
(a) Davies, H. M. L.; Hu, B.; Saikali, E.; Brazinski, P. R. J. Org. Chem. 1994, 59, 4535.
|
|
(b) Trost, B. M.; Hashmi, A. S. K. J. Am. Chem. Soc. 1994, 116, 2183.
|
|
|
(c) Hoffmann, M. Ph.D. Dissertation, Technische Universität, Dresden, 1994.
|
|
| [18] |
Arduengo III, A. J.; Harlow, R. L.; Kline, M. J. Am. Chem. Soc. 1991, 113, 361.
|
| [19] |
Moss, R. A.; Wtostowski, M.; Shen, S.; Krogh-Jespersen, K.; Matro, A. J. Am. Chem. Soc. 1988, 110, 4443.
|
| [20] |
(a) Warkentin, J. Acc. Chem. Res. 2009, 42, 205.
|
|
(b) Warkentin, J. In Advances in Carbene Chemistry, Vol. 2, Ed.:Brinker, U. H., JAI Press, Greenwich, 1998, pp. 245-295.
|
|
| [21] |
(a) Moss, R. A.; Cox, D. P. Tetrahedron Lett. 1985, 26, 1931.
|
|
(b) Olofson, R. A.; Walinsky, S. W.; Marino, J. P.; Jernow, J. L. J. Am. Chem. Soc. 1968, 90, 6554.
|
|
|
(c) Sheeren, J. W.; Slaps, R. J. F. M.; Nivard, R. J. F. Recl. Trav. Chim. Pays-Bas 1973, 92, 11.
|
|
|
(d) Corey, E. J.; Winter, R. A. E. J. Am. Chem. Soc. 1963, 85, 2677.
|
|
| [22] |
El-Saidi, M. Kassam, K.; Pole, D-L.; Tadey, T.; Warkentin, J. J. Am. Chem. Soc. 1992, 114, 8751.
|
| [23] |
Spino, C.; Rezaei, H.; Dupont-Gaudet, K.; Bélanger, F. J. Am. Chem. Soc. 2004, 126, 9926.
|
| [24] |
Boisvert, C.; Beaumier, F.; Spino, C. Org. Lett. 2007, 9, 5361.
|
| [25] |
Beaumier, F.; Dupuis, M.; Spino, C.; Legault, C.-L. J. Am. Chem. Soc. 2012, 134, 5938.
|
| [26] |
Gund, M.; Déry, M.; Amzallag, V.; Spino, C. Org. Lett. 2016, 18, 4280.
|
| [27] |
Cao, F.; Hu, F.; Xie, Q. M.; Luo, G. Y.; Chu, W. D.; He, L.; Liu, Q. Z. Asian J. Org. Chem. 2018, 7, 363.
|
| [28] |
(a) Wulff, W. D.; Yang, D. C.; Murray, C. K. Pure Appl. Chem. 1988, 60, 137.
|
|
(b) Rodriguez, K. X.; Kaltwasser, N.; Toni, T. A.; Ashfeld, B. L. Org. Lett. 2017, 19, 2482.
|
|
|
(c) Meloche, J. L.; Ashfeld, B. L. Angew. Chem., Int. Ed. 2017, 56, 6604.
|
|
| [29] |
Hu, F.; Zhou, Q.; Cao, F.; Chu, W. D.; He, L.; Liu, Q. Z. J. Org. Chem. 2018, 83, 12806.
|
| [30] |
(a) Simmons, H. E.; Smith, R. D. J. Am. Chem. Soc. 1958, 80, 5323.
|
|
(b) Simmons, H. E.; Smith, R. D. J. Am. Chem. Soc. 1959, 81, 4256.
|
|
|
(c) Simmons, H. E.; Cairns, T. L.; Vladuchick, S. A.; Hoiness, C. M. Org. React. 1973, 20, 1.
|
|
|
(d) Charette, A. B. Beauchemin, A. Org. React. 2001, 58, 1.
|
|
| [31] |
Sugita, H.; Mizuno, K.; Mori, T.; Isagawa, K.; Otsuji, Y. Angew. Chem., Int. Ed. Engl. 1991, 30, 984.
|
| [32] |
Fuchibe, K.; Aono, T.; Hu, J.; Ichikawa, J. J. Org. Lett. 2016, 18, 4502.
|
| [33] |
Zhou, Y. Y.; Uyeda, C. Science 2019, 363, 857.
|
| [34] |
Behlen, M. J.; Uyeda, C. J. Am. Chem. Soc. 2020, 142, 17294.
|
| [35] |
Yoon, T. P.; Jacobsen, E. N. Science 2003, 299, 1691.
|
| [36] |
Fischer, E. O.; Maasböl, A. Angew. Chem., Int. Ed. Engl. 1964, 3, 580.
|
| [37] |
(a) Hegedus, L. S. Tetrahedron 1997, 53, 4105.
|
|
(b) Barluenga, J.; Fañanas, F. J. Tetrahedron 2000, 56, 4597.
|
|
|
(c) Barluenga, J.; Fernandez-Rodríguez, M. A. J. Organomet. Chem. 2005, 690, 539.
|
|
|
For reviews, see: (d) Ed.: Dörwald, F. Z. Metal Carbenes in Organic Synthesis, Wiley-VCH, Weinheim, 2008.
|
|
|
(e)Metal Carbenes in Organic Synthesis, Vol. 13, Ed.: Dötz, K. H., Berlin, Springer, 2004.
|
|
|
(f) Dotz, K. H.; Stendel, J. Chem. Rev. 2009, 109, 3227.
|
|
| [38] |
Sierra, M. A.; Soderberg, B.; Lander, P. A.; Hegedus, L. S. Organo- metallics 1993, 12, 3769.
|
| [39] |
Barluenga, J.; Aznar, F.; Fernandez, M. Chem.-Eur. J. 1997, 3, 1629.
|
| [40] |
Buchert, M.; Hoffmann, M.; Reibig, H. U. Chem. Ber. 1995, 128, 605.
|
| [41] |
Barluenga, J.; Lopez, S.; Florez, J. Angew. Chem., Int. Ed. 2003, 42, 231.
|
| [42] |
(a) Hoffmann, M.; Buchert, M.; Reissig, H. U. Angew. Chem., Int. Ed. Engl. 1997, 36, 283.
|
|
(b) Hoffmann, M.; Buchert, M.; Reissig, H. U. Chem.-Eur. J. 1999, 5, 876.
|
|
| [43] |
Kagoshima, H.; Okamura, T.; Akiyama, T. J. Am. Chem. Soc. 2001, 123, 7182.
|
| [44] |
Aznar, F. Fananas-Mastral, M.; Alonso, J.; Fananas, F. J. Chem.- Eur. J. 2008, 14, 325.
|
| [45] |
Dery, M.; Lefebvre, L. P.; Aissa, K.; Spino, C. Org. Lett. 2013, 15, 5456.
|
| [46] |
(a) Horvath, R. F.; Chan, T. H. J. Org. Chem. 1987, 52, 4489.
|
|
(b) Liu, D.; Kozmin, S. A. Angew. Chem., Int. Ed. 2001, 40, 4757.
|
|
|
(c) Sen, S.; Purushotham, M.; Qi, Y.; Sieburth, S. M. Org. Lett. 2007, 9, 4963.
|
|
|
(d) Singh, S.; Sieburth, S. M. Org. Lett. 2012, 14, 4422.
|
|
|
For a review see: (e) Hermanns, J.; Schmidt, B. J. Chem. Soc., Perkin Trans. 1 1999, 81.
|
|
| [47] |
(a) Igawa, K.; Yoshihiro, D.; Abe, Y.; Tomooka, K. Angew. Chem., Int. Ed. 2016, 55, 5814.
|
|
(b) Igawa, K.; Kuroo, A.; Yoshihiro, D.; Yamanaka, Y.; Tomooka, K. Synlett 2017, 28, 2445.
|
|
|
(c) Morontsev, A.; Gringolts, M.; Lakhtin, V.; Finkelshtein, E. J. Organomet. Chem. 2020, 911, 121156.
|
|
| [48] |
Salomon, R. G. J. Org. Chem. 1974, 39, 3602.
|
| [49] |
Dunogues, J.; Calas, R.; Dedier, J.; Pisciotti, F.; Lapouyade, P. J. Organomet. Chem. 1970, 25, 51.
|
| [50] |
(a) Beteille, J. P.; Clarke, M. P.; Davidson, I. M. T.; Dubac, J. Organometallics 1989, 8, 1292.
|
|
(b) Nagao, Y.; Takahashi, M.; Abe, Y.; Misono, T.; Jung, M. E. Bull. Chem. Soc. Jpn. 1993, 66, 2294.
|
|
|
(c) Jiang, P.; Gaspar, P. P. J. Am. Chem. Soc. 2001, 123, 8622.
|
|
|
(d) Nagao, Y.; Kimura, C.; Kozawa, K.; Jung, M. E. Silicon Chem. 2003, 2, 99.
|
|
| [51] |
Richter, W. J. J. Organomet. Chem. 1985, 289, 45.
|
| [52] |
Zhang, S.; Conlin, R. T. J. Am. Chem. Soc. 1991, 113, 4272.
|
| [53] |
(a) Hwang, R.-J.; Conlin, R. T.; Gaspar, P. P. J. Organomet. Chem. 1975, 94, C38.
|
|
(b) Sakurai, H.; Kobayashi, Y.; Sato, R.; Nakadaira, Y. Chem. Lett. 1983, 12, 1197.
|
|
|
(c) Bobbitt, K. L.; Gaspar, P. P. J. Organomet. Chem. 1995, 499, 17.
|
|
| [54] |
(a) Gaspar, P. P.; Hwang, R. J. J. Am. Chem. Soc. 1974, 96, 6198.
|
|
(b) Conlin, R. T.; Peterson, L. L. J. Organomet. Chem. 1982, 232, C71.
|
|
|
(c) Lei, D.; Hwang, R.-J.; Gaspar, P. P. J. Organomet. Chem. 1984, 271, 1.
|
|
| [55] |
(a) Corriu, R.; Lanneau, G.; Priou, C.; Soulairol, F.; Auner, N.; Probst, R.; Conlin, R.; Tan, C. J. Organomet. Chem. 1994, 466, 55.
|
|
(b) Tamao, K.; Nagata, K.; Asahara, M.; Kawachi, A.; Ito, Y.; Shiro, M. J. Am. Chem. Soc. 1995, 117, 11592.
|
|
|
(c) Belzner, J.; Ihmels, H.; Kneisel, B. O.; Gould, R. O.; Herbst-Irmer, R. Organometallics 1995, 14, 305.
|
|
|
(d) Takeda, N.; Tokitoh, N.; Okazaki, R. Chem. Lett. 2000, 29, 622.
|
|
| [56] |
Toulokhonova, I. S.; Friedrichsen, D. R.; Hill, N. J.; Müller, T.; West, R. Angew. Chem., Int. Ed. 2006, 45, 2578.
|
| [57] |
Ohmura, T.; Masuda, K.; Takase, I.; Suginome, M. J. Am. Chem. Soc. 2009, 131, 16624.
|
| [58] |
Masuda, K.; Ohmura, T.; Suginome, M. Organometallics 2011, 30, 1322.
|
| [59] |
Sasaki, I.; Maebashi, A.; Li, J.; Ohmura, T.; Suginome, M. Eur. J. Org. Chem. 2022, e202101573.
|
| [60] |
Appler, H.; Neumann, W. P. J. Organomet. Chem. 1986, 314, 261.
|
| [61] |
Qi, L. L.; Pan, Q. Q.; Wei, X. X.; Pang, X. B.; Liu, Z. T.; Shu, X. Z. J. Am. Chem. Soc. 2023, 145, 13008.
|
| [62] |
Neumann, W. P. Chem. Rev. 1991, 91, 311.
|
| [63] |
Lei, D. Q.; Gaspar, P. P. Polyhedron 1991, 10, 1221.
|
| [64] |
Sasamori, T.; Miyamoto, H.; Sakai, H.; Furukawa, Y.; Tokitoh, N. Organometallics 2012, 31, 3904.
|
| [65] |
(a) Dequirez, G.; Pons, V.; Dauban, P. Angew. Chem., Int. Ed. 2012, 51, 7384.
|
|
(b) Ju, M.; Schomaker, J. M. Nat. Rev. Chem. 2021, 5, 580.
|
|
|
(c) Wang, Y. C.; Lai, X. J.; Huang, K. K.; Yadav, S.; Qiu, G. Y. S.; Zhang, L. P.; Zhou, H. W. Org. Chem. Front. 2021, 8, 1677.
|
|
| [66] |
Wu, Q.; Hu, J.; Ren, X. F.; Zhou, J. R. Chem.-Eur. J. 2011, 17, 11553.
|
| [67] |
Brichacek, M.; Villalobos, M.; Plichta, N. A.; Njardarson, J. T. Org. Lett. 2011, 13, 1110.
|
| [68] |
(a) Mimura, N.; Ibuka, T.; Akaji, M.; Miwa, Y.; Taga, T.; Nakai, K.; Tamamura, H.; Fujii, N.; Yamamoto, Y. Chem. Commun. 1996, 351.
|
|
(b) Ibuka, T.; Mimura, N.; Aoyama, H.; Akaji, M.; Ohno, H.; Miwa, Y.; Taga, T.; Nakai, K.; Tamamura, H.; Fujii, N.; Yamamoto, Y. J. Org. Chem. 1997, 62, 999.
|
| [1] | 王晓梅, 刘岩, 李师伍, 赵志飞. 氮杂环卡宾(NHC)催化水杨醛与吡唑啉酮-4,5-二酮的[4+2]环加成反应高效构建螺缩酮-吡唑啉酮化合物[J]. 有机化学, 2025, 45(1): 267-275. |
| [2] | 蒋镓西, 刘全忠. 乙烯基重氮化合物非金属卡宾机制参与的反应[J]. 有机化学, 2024, 44(9): 2640-2657. |
| [3] | 赵明, 颜瑞, 陈虎. 氮杂环卡宾催化醛类化合物的极性反转[J]. 有机化学, 2024, 44(7): 2204-2215. |
| [4] | 孙超, 周泉, 李传莹, 王磊. 苯并唑-氮杂环卡宾钯配合物的合成表征及应用[J]. 有机化学, 2024, 44(6): 1957-1966. |
| [5] | 刘晓东, 施世良. ANIPE配体促进的铜催化联烯与亚胺和联硼试剂的不对称碳硼化反应[J]. 有机化学, 2024, 44(6): 1884-1896. |
| [6] | 徐光利, 韩鸿萍, 曹露微, 洪思敏, 海林悦, 崔香. 过渡金属催化1,3-共轭二烯基硼化合物合成研究进展[J]. 有机化学, 2024, 44(5): 1480-1493. |
| [7] | 刘岩, 王晓梅, 何林, 李师伍, 赵志飞. 氮杂环卡宾(NHC)催化[3+2]环加成反应高非对映选择性地构建螺氧吲哚二氢呋喃稠合吡唑啉酮化合物[J]. 有机化学, 2024, 44(4): 1301-1310. |
| [8] | 孔德亮, 杨萧昂, 赵怡玲, 彭彦博, 朱红平. 硅宾与质子氢分子的氧化加成反应合成硅氢物种[J]. 有机化学, 2024, 44(4): 1311-1318. |
| [9] | 夏登鹏, 罗锦昀, 何林, 蔡志华, 杜广芬. 氮杂环卡宾催化的五氟苯基硫醚的合成[J]. 有机化学, 2024, 44(2): 622-630. |
| [10] | 杨爽, 房新强. 氮杂环卡宾催化实现的动力学拆分近期研究进展[J]. 有机化学, 2024, 44(2): 448-480. |
| [11] | 杜友龙, 王倩, 梅海波, Romana Pajkert, Gerd-Volker Röschenthaler, 韩建林. α,α-二氟-β-氨基膦酸酯的合成与应用研究进展[J]. 有机化学, 2024, 44(12): 3686-3701. |
| [12] | 孙庆浩, 鲍晓光. 钼催化芳香醛脱氧偶联反应机制的理论研究[J]. 有机化学, 2024, 44(11): 3518-3525. |
| [13] | 田雁, 董睿, 聂鹏, 许波. 含不同取代基的钌-锗化合物的合成及表征[J]. 有机化学, 2024, 44(1): 173-179. |
| [14] | 蔡远林, 吕亚, 聂桂花, 金智超, 池永贵. 氮杂环卡宾催化合成氰基化合物的研究进展[J]. 有机化学, 2023, 43(9): 3135-3145. |
| [15] | 王玉超, 刘晋彪, 何智涛. 钯催化共轭二烯的不对称氢官能团化[J]. 有机化学, 2023, 43(8): 2614-2627. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||