有机化学 ›› 2025, Vol. 45 ›› Issue (9): 3175-3185.DOI: 10.6023/cjoc202506006 上一篇 下一篇
综述与进展
赵友学†, 李兮若†, 孟洛冰, 李春秀, 范贵生, 许建和*( )
)
收稿日期:2025-06-03
修回日期:2025-08-21
发布日期:2025-09-11
作者简介:† 共同第一作者
基金资助:
Youxue Zhao, Xiruo Li, Luobing Meng, Chunxiu Li, Guisheng Fan, Jianhe Xu*( )
)
Received:2025-06-03
Revised:2025-08-21
Published:2025-09-11
Contact:
E-mail: About author:† The authors contributed equally to this work.
★ Academic Papers of the 27th Annual Meeting of the China Association for Science and Technology.
Supported by:文章分享

手性醇作为药物活性分子与精细化学品的关键手性砌块, 在医药、农药及化工等领域具有广泛应用. 然而传统合成技术存在效率低、选择性不足等瓶颈, 亟需通过新型催化体系实现高效不对称合成. 醇脱氢酶(ADHs)/羰基还原酶(CRs)作为手性醇绿色合成的核心生物催化剂, 其分子设计与工业应用已成为研究热点. 尽管这类酶具有环境友好性和催化特异性的优势, 但目前仍受限于底物宽泛性不足、底物适配性差等问题, 严重制约了其在手性醇精准合成中的规模化应用. 此综述系统阐述了DHs/CRs多底物适配度定量研究最新进展, 重点围绕酶库与多底物间构效关系的解析方法展开讨论, 并结合当前研究趋势提出未来发展方向. 期望为研究者提供理论参考, 推动多酶-多底物构效关系的深度探索, 进而建立ADHs/CRs的智能预测与设计模型, 实现手性醇合成用酶从“大海捞针式筛选”到“精准定制化开发”的技术革新.
赵友学, 李兮若, 孟洛冰, 李春秀, 范贵生, 许建和. 醇脱氢酶/羰基还原酶与多底物分子适配性研究的进展★[J]. 有机化学, 2025, 45(9): 3175-3185.
Youxue Zhao, Xiruo Li, Luobing Meng, Chunxiu Li, Guisheng Fan, Jianhe Xu. Advances in Understanding the Substrate Promiscuity of Alcohol Dehydrogenases/Carbonyl Reductases★[J]. Chinese Journal of Organic Chemistry, 2025, 45(9): 3175-3185.






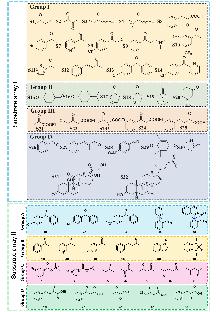



| [1] |
|
|
(江杏杏, 武卫龙, 任慧莹, 张凤, 莫文枝, 卢志强, 化学学报, 2024, 82, 736.)
|
|
| [2] |
|
| [3] |
|
|
(张文鹤, 祝天慧, 秦凤玉, 游松, 生物产业技术, 2019, 3, 54.)
|
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
doi: 10.1007/s00018-008-8588-y pmid: 19011750 |
| [9] |
doi: 10.1039/d3ob01447a pmid: 38050738 |
| [10] |
doi: 10.1021/bi00018a001 pmid: 7742302 |
| [11] |
|
|
(张晓健, 刘倩, 柳志强, 郑裕国, 化工进展, 2021, 40, 1142.)
|
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [1] | 秦丽清, 林桂汕, 段文贵, 崔玉成, 杨卯芳, 李芳耀, 李典鹏. 新型长叶烯基萘满并N-酰基吡唑化合物的合成、抗增殖活性、三维定量构效关系及分子对接研究[J]. 有机化学, 2024, 44(6): 1967-1977. |
| [2] | 崔玉成, 陈美桦, 林桂汕, 段文贵, 李晴敏, 邹壬萱, 岑波. 含偕二甲基环丙烷结构的1,3,4-噻二唑-脲化合物的合成、抑菌活性及分子对接研究[J]. 有机化学, 2022, 42(11): 3784-3797. |
| [3] | 徐洪亮, 苏静, 王子时, 侯晨忻, 吴鹏冲, 邢月, 李香帅, 朱晓磊, 路运才, 徐利剑. 苯基吡咯类杀菌剂的设计合成及三维-定量构效关系(3D-QSAR)研究[J]. 有机化学, 2021, 41(9): 3560-3570. |
| [4] | 李成飞, 岑波, 段文贵, 林桂汕, 王秀, 李宝谕. 含天然苯乙烯结构的4-甲基-1,2,4-三唑-硫醚化合物的合成、除草活性及三维定量构效关系(3D-QSAR)研究[J]. 有机化学, 2021, 41(6): 2485-2495. |
| [5] | 虞友培, 段文贵, 林桂汕, 康国强, 王晓宇, 雷福厚. 新型含偕二甲基环丙烷的4-甲基-1,2,4-三唑硫醚化合物的合成、生物活性及三维定量构效关系(3D-QSAR)研究[J]. 有机化学, 2020, 40(6): 1647-1657. |
| [6] | 崔紫宁, 张莉, 黄娟, 凌云, 杨新玲. 含呋喃环双酰肼类衍生物的合成、杀虫活性及3D-QSAR研究[J]. 有机化学, 2010, 30(10): 1482-1491. |
| [7] | 朱有全; 刘卫敏; 刘 斌 ; 胡方中 朱 然; 邹小毛* ; 杨华铮* . 具有除草活性的3-(嘧啶基-2)-1,3,4-噁二唑-2(3H)-酮衍生物的设计、 合成与定量构效关系的研究[J]. 有机化学, 2009, 29(04): 638-642. |
| [8] | 朱有全,成佳,邹小毛,胡方中,肖玉虹,杨华铮. 具有除草活性的吡唑啉并四嗪酮衍生物的设计、合成与定量构效关系的研究[J]. 有机化学, 2008, 28(06): 1044-1049. |
| [9] | 朱有全,邹小毛,李公春,姚昌盛,司学凯,杨华铮. 3-取代苄基-6-三氟甲基嘧啶-2,4(1H,3H)-二酮衍生物的合成及其除草活性的研究[J]. 有机化学, 2007, 27(06): 753-757. |
| [10] | 杨道武, 余训民. 新定义的价连接性指数与有机物理化活性的相关性研究[J]. 有机化学, 2004, 24(5): 525-536. |
| [11] | 沐来龙, 冯长君. 脂肪族饱和一元醇沸点的拓扑研究[J]. 有机化学, 2004, 24(4): 403-408. |
| [12] | 沐来龙, 冯长君. 不饱和链烃沸点的拓扑研究[J]. 有机化学, 2004, 24(2): 173-183. |
| [13] | 吴斌, 李敏勇, 江振洲, 夏霖. N-取代-3-吲哚乙酰胺类α1-肾上腺素受体拮抗剂的设计、合成及其3D-QSAR研究[J]. 有机化学, 2004, 24(12): 1587-1594. |
| [14] | 秦正龙,冯长君. 取代苯酚的定量结构-活性/性质相关性研究[J]. 有机化学, 2003, 23(7): 654-658. |
| [15] | 周文富,孙贺琦. 硝基苯类化合物的FMO位电荷密度能S_(Ei)~(HO)与生物毒性的QSAR研究[J]. 有机化学, 2003, 23(7): 705-709. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||