有机化学 ›› 2026, Vol. 46 ›› Issue (2): 315-355.DOI: 10.6023/cjoc202509021 上一篇 下一篇
综述与进展
收稿日期:2025-09-18
修回日期:2025-10-01
发布日期:2025-10-23
通讯作者:
高峰
基金资助:
Jing Lia,b, Feng Gaoa,*( ), Wanbin Zhanga
), Wanbin Zhanga
Received:2025-09-18
Revised:2025-10-01
Published:2025-10-23
Contact:
Feng Gao
Supported by:文章分享
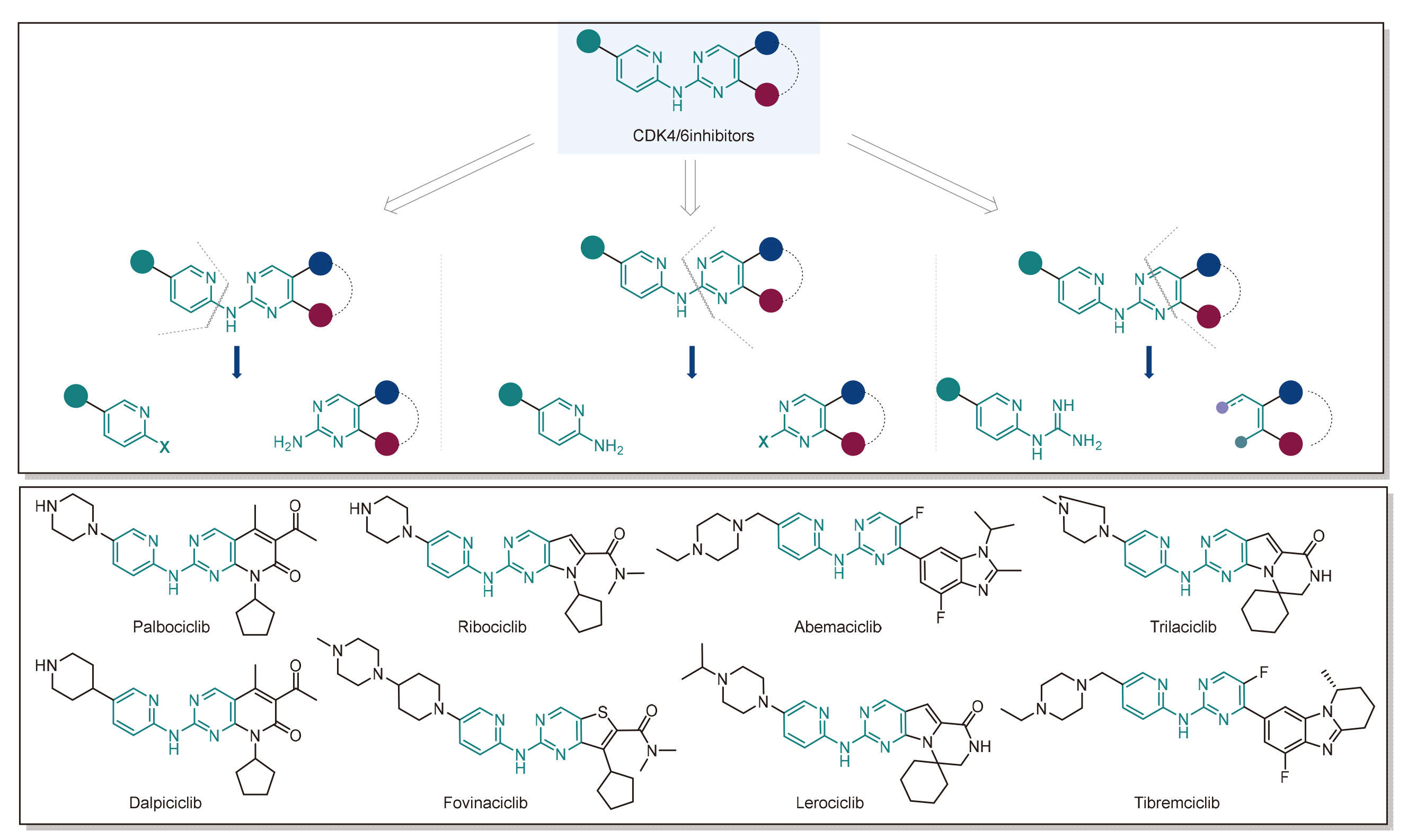
CDK4/6抑制剂作为第三代细胞周期蛋白依赖性激酶抑制剂, 凭借其低毒性和高体内活性的优势, 已成为治疗乳腺癌、肺癌等癌症的关键药物. 此综述结合药物上市时间与结构相似性, 系统评述哌柏西利与达尔西利、瑞波西利与伏维西利、阿贝西利与泰瑞西利、曲拉西利与来罗西利等8种代表性药物的合成路径, 重点分析新反应体系与合成策略对工艺效率的提升作用. 这类分子多以N-(吡啶-2-基)嘧啶-2-胺为核心骨架, 其合成依赖金属催化交叉偶联(C—N键构建)与缩合反应(嘧啶骨架构建), 面临区域选择性控制难、贵金属依赖高和工艺放大性差等挑战. 因此, 开发绿色、经济和高效的新型合成工艺, 是该领域未来核心优化方向.
李静, 高峰, 张万斌. 抗肿瘤药物CDK4/6抑制剂合成工艺研究进展[J]. 有机化学, 2026, 46(2): 315-355.
Jing Li, Feng Gao, Wanbin Zhang. Advances in the Synthesis Process of Antitumor Drugs CDK4/6 Inhibitors[J]. Chinese Journal of Organic Chemistry, 2026, 46(2): 315-355.
| [1] |
|
| [2] |
a)
|
|
(b)
|
|
|
(c)
|
|
|
(d)
|
|
| [3] |
a)
|
|
(b)
|
|
|
(c)
|
|
|
(d)
|
|
|
(e)
|
|
|
(f)
|
|
|
(g)
|
|
|
(h)
|
|
|
(i)
|
|
| [4] |
a)
|
|
(b)
|
|
|
(c)
|
|
|
(d)
|
|
| [5] |
a)
|
|
(b)
|
|
| [6] |
a)
|
|
(b)Novartis Annual Results 2024, 2025, https://www.novartis.com/investors/financial-data/annual-results(accessed 2025-01-31).
|
|
|
(c)Eli Lilly and Company 2024 Annual Report, 2024, https://investor.lilly.com/financial-information/annual-reports(accessed 2024-12-31).
|
|
| [7] |
a) National Medical Products Administration, 2021, https://www.nmpa.gov.cn/directory/web/nmpa/zhuanti/ypqxgg/gggzjzh/20211231170049193.html(accessed 2021-12-31).
|
|
(b) National Medical Products Administration, 2025, https://www.nmpa.gov.cn/zhuanti/cxylqx/cxypxx/20250529152341165.html(accessed 2025-5-29).
|
|
|
(c)
|
|
|
(d) National Medical Products Administration, 2025, https://www.nmpa.gov.cn/zhuanti/cxylqx/cxypxx/20250529152115100.html(accessed 2025-05-29).
|
|
|
(f) National Medical Products Administration, 2025, https://www.nmpa.gov.cn/zhuanti/cxylqx/cxypxx/20250702173549141.html(accessed 2025-07-02).
|
|
|
(g)
|
|
| [1] |
|
| [2] |
a)
|
|
(b)
|
|
|
(c)
|
|
| [3] |
|
| [4] |
a)
|
|
(b) Shagufta; Ahmad, I.; Bazbouz, L. Z.; Nasar, N. A.; Ibrahim, F. G.; Alkheder, D. M. Med. Chem. Res. 2025, 34, 1237.
|
|
| [5] |
a)
|
|
(b)
|
|
|
(c)
|
|
|
(d)
|
|
|
(e)
|
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
a)
|
|
(b)
|
|
|
(c)
|
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
|
| [31] |
|
| [32] |
|
| [33] |
|
| [34] |
|
| [35] |
|
| [36] |
|
| [37] |
|
| [38] |
|
| [39] |
|
| [40] |
|
| [41] |
|
| [42] |
|
| [43] |
|
| [44] |
|
| [45] |
|
| [46] |
|
| [47] |
|
| [48] |
|
| [49] |
|
| [50] |
|
| [51] |
|
| [52] |
|
| [53] |
|
| [54] |
|
| [55] |
|
| [56] |
|
| [57] |
|
| [58] |
|
| [59] |
|
| [60] |
|
| [61] |
|
| [62] |
|
| [63] |
|
| [64] |
|
| [65] |
|
| [66] |
|
| [67] |
|
| [68] |
|
| [69] |
|
| [70] |
|
| [71] |
|
| [72] |
|
| [73] |
|
| [74] |
|
| [75] |
GlobeNewswire, FDA Approves G1 Therapeutics’ COSELA™ (trilaciclib): The First and Only Myeloprotection Therapy to Decrease the Incidence of Chemotherapy-Induced Myelosuppression. 2021, https://www.globenewswire.com/news-release/2021/02/13/ 2175184/0/en/FDA-Approves-G1-Therapeutics-COSELA-trilaciclib-The-First-and-Only-Myeloprotection-Therapy-to-Decrease-the-Incidence-of-Chemotherapy-Induced-Myelosuppression.html (acce-ssed 2021-2-13).
|
| [76] |
|
| [77] |
|
| [78] |
|
| [79] |
|
| [80] |
|
| [81] |
|
| [82] |
|
| [83] |
|
| [84] |
Shanghai Tianci Bio Valley Biological Engineering Co., Ltd, CN 116693535 A, 2022.
|
| [85] |
|
| [86] |
|
| [87] |
|
| [7] |
(e)
|
| [1] | 刘荣, 左应林, 张英俊, 张霁. “精准化学”在药物发现中的应用★[J]. 有机化学, 2025, 45(9): 3075-3097. |
| [2] | 郭书洋, 何雨蒙, 程小敏, 高宇星, 张玉琦, 马豪杰, 何卫民. 电化学多组分串联合成4-硒基酰基吡唑[J]. 有机化学, 2025, 45(10): 3807-3815. |
| [3] | 夏坤, 张开发, Sher Wali Khan, 阿布力米提•阿布都卡德尔. 二氧化碳参与的三组分偶联反应进展[J]. 有机化学, 2024, 44(5): 1506-1525. |
| [4] | 黄净, 杨毅华, 张占辉, 刘守信. 酰胺键的绿色高效构建方法与技术进展[J]. 有机化学, 2024, 44(2): 409-420. |
| [5] | 赵永哲, 孔翔飞, 张俊良, 杨俊锋. 含膦负载催化剂的合成及应用研究进展[J]. 有机化学, 2024, 44(12): 3587-3608. |
| [6] | 梁金少, 阮丽红, 石栩溶, 陈樱枝, 宋楚焕, 肖军安, 刘志平. 机械力化学促进给-受体环丙烷与吲哚-2-二芳甲醇傅-克烷基化反应[J]. 有机化学, 2024, 44(11): 3437-3445. |
| [7] | 岁丹丹, 岑南楠, 龚若蕖, 陈阳, 陈文博. 无支持电解质条件下连续流电化学合成三氟甲基化氧化吲哚[J]. 有机化学, 2023, 43(9): 3239-3245. |
| [8] | 蒋宜欣, 唐伯孝, 毛海波, 陈雪霞, 俞洋杰, 全翠英, 徐昭阳, 石金慧, 刘益林. 水-聚乙二醇(PEG-200)中烯烃与碘代芳烃绿色可循环无负载偶联反应的研究[J]. 有机化学, 2023, 43(9): 3210-3215. |
| [9] | 卢凯, 屈浩琦, 陈樨, 秋慧, 郑晶, 马猛涛. 无催化剂、无溶剂条件下炔烃和烯烃与儿茶酚硼烷的硼氢化反应[J]. 有机化学, 2023, 43(6): 2197-2205. |
| [10] | 莫百川, 陈春霞, 彭进松. 木质素及其衍生物负载金属催化剂在有机合成中的应用研究进展[J]. 有机化学, 2023, 43(4): 1215-1240. |
| [11] | 窦谦, 汪太民, 房丽晶, 翟宏斌, 程斌. 光诱导铁催化在有机合成中的应用研究进展[J]. 有机化学, 2023, 43(4): 1386-1415. |
| [12] | 李奇阳, 张海燕, 刘文博. 无过渡金属参与的碳硅键构筑方法研究进展[J]. 有机化学, 2023, 43(10): 3470-3490. |
| [13] | 宇世伟, 陈兆华, 陈淇, 林舒婷, 何金萍, 陶冠燊, 汪朝阳. 硫代磺酸酯的合成与应用研究进展[J]. 有机化学, 2022, 42(8): 2322-2330. |
| [14] | 顾清云, 程振凤, 曾小宝. 电化学氧化三氟甲基亚磺酸钠与α-羰基二硫缩烯酮的三氟甲基化反应[J]. 有机化学, 2022, 42(5): 1537-1544. |
| [15] | 郑煜, 钱沈城, 徐鹏程, 郑斌南, 黄申林. 电化学氧化芳基端炔的硫氰化磺化反应[J]. 有机化学, 2022, 42(12): 4275-4281. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||