有机化学 ›› 2011, Vol. 31 ›› Issue (10): 1618-1623. 上一篇 下一篇
研究论文
吴兆军1,李顺来1,丁忠仁2,杜洪光*,1
Wu Zhaojun1 Li Shunlai1 Ding Zhon-gren2 Du Hongguang*,1
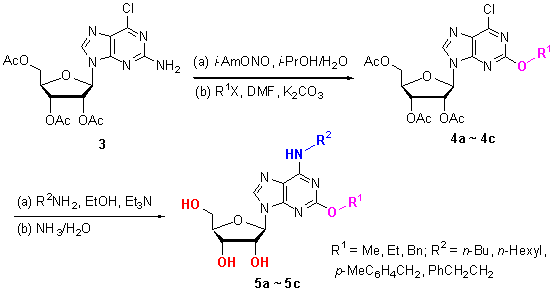
以鸟嘌呤核苷(1)为原料, 经羟基保护得到2,3,5-三-O-乙酰基鸟嘌呤核苷(2), 2与三氯氧磷反应得到2-氨基-6-氯-9-(2,3,5-三-O-乙酰基-β-D-呋喃核糖)嘌呤(3), 3经重氮化、水解和O-烷基化得到2-烷氧基-6-氯-9-(2,3,5-三-O-乙酰基-β-D-呋喃核糖)嘌呤(4a~4c), 4a~4c经胺解和水解脱保护反应得到12个未见报道的N6-烷基-2-烷氧基腺苷化合物5a~5c. 化合物的结构经1H NMR, 13C NMR, IR和HRMS等得到表征, 同时对合成的N6-烷基-2-烷氧基腺苷化合物进行了抗血小板凝集活性测试. 结果表明, 在测试浓度为100 μmol/L时, N6-(4-甲基苄基)-2-苄氧基腺苷(5c3)和N6-(2-苯乙基)-2-苄氧基腺苷(5c4)具有相对较低的聚集率, 具有一定的抗血小板凝集活性.