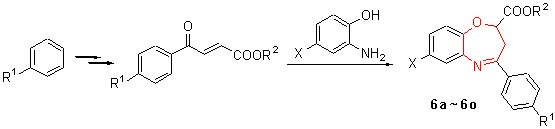
| [1] Chakravarti, B.; Siddiqui, J. A.; Dwivedi, S. K. D.; Deshpande, S.; Samanta, K.; Bhatta, R. S.; Panda, G.; Prabhaka, Y. S.; Konwar, R.; Sanyal, S.; Chattopadhyay, N. Mol. Cell. Endocrinol. 2011, 338, 68. [2] Maginn, E. N.; Browne, P. V.; Hayden, P.; Vandenberghe, E.; MacDonagh, B.; Evans, P.; Goodyer, M.; Tewari, P.; Campiani, G.; Butini, S.; Williams, D. C.; Zisterer, D. M.; Lawler, M. P.; McElligott, A. M. Br. J. Cancer 2011, 104, 281. [3] Zificsak, C. A.; Theroff, J. P.; Aimone, L. D.; Albom, M. S.; Angeles, T. S.; Brown, R. A.; Galinis, D.; Grobelny, J. V.; Herbertz, T.; Husten, J.; Kocsis, L. S.; LoSardo, C.; Miknyoczki, S. J.; Murthy, S.; Rolon-Steele, D.; Underiner, T. L.; Wells-Knecht, K. J.; Worrell, C. S.; Zeigler, K. S.; Dorsey, B. D. Bioorg. Med. Chem. Lett. 2011, 21, 660. [4] L髉ez-Cara, L. C.; Conejo-Garc韆, A.; Marchal, J. A.; Macchione, G.; Cruz-L髉ez, O.; Boulaiz, H.; Garc韆, M. A.; Rodr韌uez- Serrano, F.; Ram韗ez, A.; Cativiela, C.; Jim閚ez, A. I.; Garc韆-Ruiz, J. M.; Choquesillo-Lazarte, D.; Ar醤ega, A.; Campos, J. M. Eur. J. Med. Chem. 2011, 46, 249. [5] Bajaj, K.; Archana; Kumar, A. Eur. J. Med. Chem. 2004, 39, 369. [6] Chandrasekhar, S.; Seenaiah, M.; Kumar, A.; Reddy, C. R.; Mamidyala, S. K.; Kumar, C. G.; Balasubramanian, S. Tetrahedron Lett. 2011, 52, 806. [7] Matsumoto, Y.; Tsuzuki, R.; Matsuhisa, A.; Yoden, T.; Yamagiwa, Y.; Yanagisawa, I.; Shibanuma, T.; Nohira, H. Bioorg. Med. Chem. 2000, 8, 393. [8] Kamei, K.; Maeda, N.; Nomura, K.; Shibata, M.; Ogino, R. K.; Koyama, M.; Nakajima, M.; Inoue, T.; Ohno, T.; Tatsuoka, T. Bioorg. Med. Chem. 2006, 14, 1978. [9] Miki, T.; Kori, M.; Fujishima, A.; Mabuchi, H.; Tozawa, R.; Nakamura, M.; Sugiyama, Y.; Yukimasa, H. Bioorg. Med. Chem. 2002, 10, 385. [10] Miki, T.; Kori, M.; Mabuchi, H.; Banno, H.; Tozawa, R.; Nakamura, M.; Itokawa, S.; Sugiyama, Y.; Yukimasa, H. Bioorg. Med. Chem. 2002, 10, 401. [11] Miki, T.; Kori, M.; Tozawa, R.; Nakamura, M.; Sugiyama, Y.; Yukimasa, H. Chem. Pharm. Bull. 2002, 50(1), 53. [12] Ichikawa, M.; Yokomizo, A.; Itoh, M.; Sugita, K.; Usui, H.; Shimizu, H.; Suzuki, M.; Terayama, K.; Kanda, A. Bioorg. Med. Chem. 2011, 19, 1930. |