有机化学 ›› 2023, Vol. 43 ›› Issue (2): 622-628.DOI: 10.6023/cjoc202206033 上一篇 下一篇
研究论文
收稿日期:2022-06-19
修回日期:2022-09-29
发布日期:2022-11-07
Tingting Liua,b, Yucai Hua, An Shena( )
)
Received:2022-06-19
Revised:2022-09-29
Published:2022-11-07
Contact:
*E-mail: 文章分享

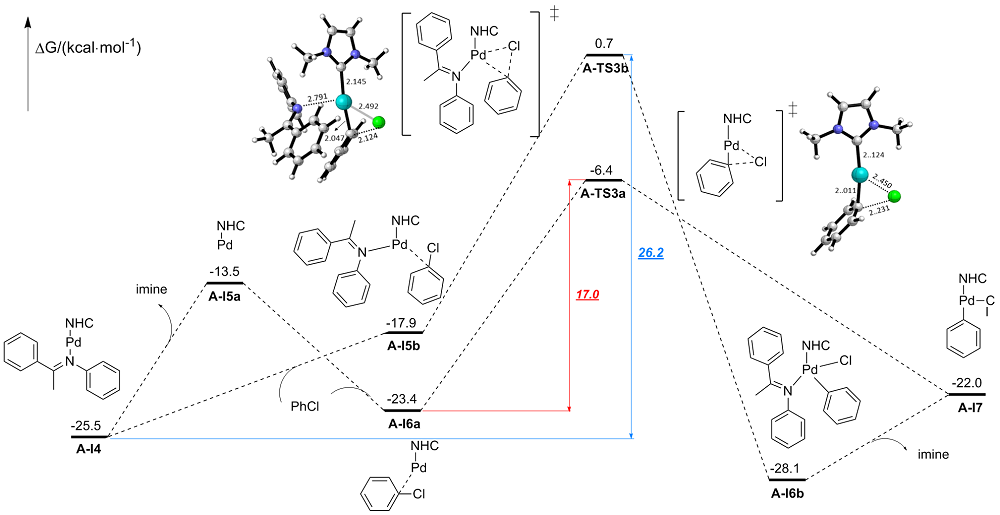
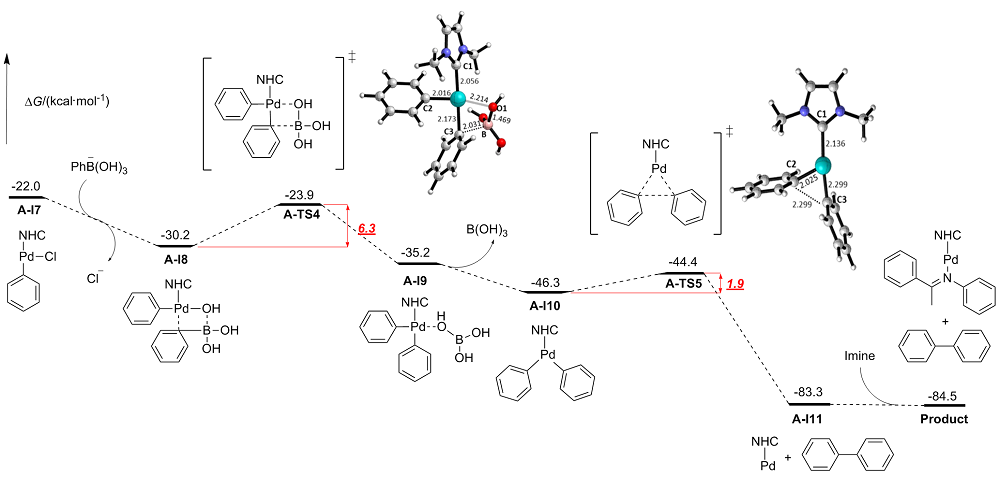
在氮杂环卡宾-钯(NHC-Pd)配合物催化的碳-碳偶联反应中, 配体结构对催化反应起到关键作用. 为了实现更好的催化效果, 不仅要对NHC配体进行结构优化, 还需要第二配体的协同作用. 然而, 由于第二配体配位能力大大弱于NHC配体, 其在催化反应中的协同作用往往被忽视. 合成出了3种由相同结构NHC配体以及不同结构亚胺配体组成的NHC-Pd配合物来催化Suzuki-Miyaura交叉偶联反应. 实验结果表明, 在相同的反应条件下, 3种亚胺配体协同的NHC-Pd配合物表现出了明显不同的催化效果. 进一步采用理论计算来深入研究亚胺配体协同的NHC-Pd配合物在催化反应中的作用机制. 通过完整催化循环过程的计算, 发现虽然亚胺配体并没有参与到催化循环过程中, 但亚胺配体与氯苯会形成竞争配位, 它们与NHC-Pd(0)的配位能力差异直接导致实际参与催化循环的有效活性中心的浓度的差异, 计算表明大位阻缺电子的亚胺配体更有利于反应的进行. 通过研究, 更好地阐明了亚胺配体在催化碳-碳偶联反应中的作用机制, 并为NHC-Pd配合物的结构调控提供了新策略.
刘婷婷, 胡宇才, 沈安. 亚胺配体协同氮杂环卡宾钯配合物催化碳碳偶联反应的作用机制[J]. 有机化学, 2023, 43(2): 622-628.
Tingting Liu, Yucai Hu, An Shen. Mechanism of Carbon-Carbon Coupling Reactions Catalyzed by Imine-Ligand-Assisted N-Heterocyclic Carbene Palladium Complexes[J]. Chinese Journal of Organic Chemistry, 2023, 43(2): 622-628.
| Entry | 21 | 23 | NHC-Pd | Yield/% | ||
|---|---|---|---|---|---|---|
| 1 | | | A B C | 64 90 97 | ||
| 2 | | | A B C | 89 96 96 | ||
| 3 | | | A B C | 93 96 >99 | ||
| 4 | | | A B C | 95 >99 >99 | ||
| 5 | | | A B C | 25 71 88 | ||
| Entry | 21 | 23 | NHC-Pd | Yield/% | ||
|---|---|---|---|---|---|---|
| 1 | | | A B C | 64 90 97 | ||
| 2 | | | A B C | 89 96 96 | ||
| 3 | | | A B C | 93 96 >99 | ||
| 4 | | | A B C | 95 >99 >99 | ||
| 5 | | | A B C | 25 71 88 | ||
| Intermediate/ transition state | NHC-Pd A | NHC-Pd B | NHC-Pd C | |||||
|---|---|---|---|---|---|---|---|---|
| GAS | SMD | GAS | SMD | GAS | SMD | |||
| I1 | -4.2 | -0.1 | -3.7 | 0.6 | -5.6 | -0.6 | ||
| TS1 | 21.5 | 20.0 | 20.6 | 20.8 | 20.9 | 21.0 | ||
| ΔG≠(TS1) | 25.7 | 20.1 | 24.3 | 20.2 | 26.5 | 21.6 | ||
| I2 | 0.5 | 3.1 | 0.0 | 3.4 | 0.7 | 4.5 | ||
| I3 | -11.8 | -12.9 | -11.3 | -12.5 | -10.3 | -11.2 | ||
| TS2 | 3.6 | 5.2 | 3.2 | 4.8 | 3.7 | 6.2 | ||
| ΔG≠(TS2) | 15.3 | 18.1 | 14.6 | 17.3 | 14.0 | 17.4 | ||
| I4 | -25.5 | -25.5 | -23.5 | -22.1 | -18.0 | -13.8 | ||
| I5a | -11.5 | -13.5 | -11.2 | -13.8 | -11.7 | -13.9 | ||
| I5a-I4 | - | 12 | - | 8.3 | - | -0.1 | ||
| I6a | -23.0 | -23.4 | -22.7 | -23.7 | -23.2 | -23.8 | ||
| TS3a | -9.6 | -6.4 | -9.4 | -6.7 | -9.8 | -6.8 | ||
| ΔG≠(TS3a) | 13.4 | 17.0 | 13.4 | 17.0 | 13.4 | 17.0 | ||
| I7 | -14.6 | -22.0 | -14.3 | -22.3 | -14.8 | -22.5 | ||
| Intermediate/ transition state | NHC-Pd A | NHC-Pd B | NHC-Pd C | |||||
|---|---|---|---|---|---|---|---|---|
| GAS | SMD | GAS | SMD | GAS | SMD | |||
| I1 | -4.2 | -0.1 | -3.7 | 0.6 | -5.6 | -0.6 | ||
| TS1 | 21.5 | 20.0 | 20.6 | 20.8 | 20.9 | 21.0 | ||
| ΔG≠(TS1) | 25.7 | 20.1 | 24.3 | 20.2 | 26.5 | 21.6 | ||
| I2 | 0.5 | 3.1 | 0.0 | 3.4 | 0.7 | 4.5 | ||
| I3 | -11.8 | -12.9 | -11.3 | -12.5 | -10.3 | -11.2 | ||
| TS2 | 3.6 | 5.2 | 3.2 | 4.8 | 3.7 | 6.2 | ||
| ΔG≠(TS2) | 15.3 | 18.1 | 14.6 | 17.3 | 14.0 | 17.4 | ||
| I4 | -25.5 | -25.5 | -23.5 | -22.1 | -18.0 | -13.8 | ||
| I5a | -11.5 | -13.5 | -11.2 | -13.8 | -11.7 | -13.9 | ||
| I5a-I4 | - | 12 | - | 8.3 | - | -0.1 | ||
| I6a | -23.0 | -23.4 | -22.7 | -23.7 | -23.2 | -23.8 | ||
| TS3a | -9.6 | -6.4 | -9.4 | -6.7 | -9.8 | -6.8 | ||
| ΔG≠(TS3a) | 13.4 | 17.0 | 13.4 | 17.0 | 13.4 | 17.0 | ||
| I7 | -14.6 | -22.0 | -14.3 | -22.3 | -14.8 | -22.5 | ||
| [1] |
Park, Y.; Kim, Y.; Chang, S. Chem. Rev. 2017, 117, 247.
doi: 10.1021/acs.chemrev.6b00826 |
| [2] |
Xia, Y.; Qiu, D.; Wang J. B. Chem. Rev. 2017, 117, 13810.
doi: 10.1021/acs.chemrev.7b00382 |
| [3] |
Bolm, C.; Hildebrand, J. P.; Muñiz, K.; Hermanns, N. Angew. Chem., Int. Ed. 2001, 40, 3284.
|
| [4] |
Andrea, B.; Paolo, C.; Alessandro, D. Z.; Marco, Z. Chem. Rev. 2018, 118, 2249.
doi: 10.1021/acs.chemrev.7b00443 pmid: 29460627 |
| [5] |
Begur, V. V.; Jayaraman, D.; Kiran, R. B.; Yogesh, S.; Kaliyamoorthy, A.; Kandikere, R. P. Tetrahedron Lett. 2017, 58, 803.
doi: 10.1016/j.tetlet.2017.01.035 |
| [6] |
Zhang, T. X.; Li, Z. Comput. Theor. Chem. 2013, 1016, 28.
doi: 10.1016/j.comptc.2013.04.015 |
| [7] |
Qian, H.; Yin, Z.; Zhang, T.; Yan, S.; Wang, Q.; Zhang, C. Organometallics 2014, 33, 6241.
doi: 10.1021/om5008924 |
| [8] |
Braga, A. A. C.; Morgon, N. H.; Ujaque, G.; Lledós, A.; Maseras, F. J. Organomet. Chem. 2006, 691, 4459.
doi: 10.1016/j.jorganchem.2006.02.015 |
| [9] |
Kozuch, S.; Amatore, C.; Jutand, A.; Shaik, S. Organometallics 2005, 24, 2319.
doi: 10.1021/om050160p |
| [10] |
Proutiere, F.; Lyngvi, E.; Aufiero, M.; Sanhueza, I. A.; Schoenebeck, F. Organometallics 2014, 33, 6879.
doi: 10.1021/om5009605 |
| [11] |
Raders, S. M.; Moore, J. N.; Parks, J. K.; Miller, A. D. J. Org. Chem. 2013, 78, 4649.
doi: 10.1021/jo400435z |
| [12] |
Wang, S. Tetrahedron Lett. 1997, 38, 5575.
doi: 10.1016/S0040-4039(97)01261-6 |
| [13] |
Littke, A. F.; Dai, C.; Fu, G. C. J. Am. Chem. Soc. 2000, 122, 4020.
doi: 10.1021/ja0002058 |
| [14] |
Kataoka, N.; Shelby, Q.; Stambuli, J. P.; Hartwig, J. F. J. Org. Chem. 2002, 67, 5553.
doi: 10.1021/jo025732j |
| [15] |
Jensen, J. F.; Johannsen, M. Org. Lett. 2003, 5, 3025.
pmid: 12916972 |
| [16] |
Yee, K. F.; Chan, K. S.; Hung, Y. C.; Chan, A. S. C. Chem. Commun. 2004, 36, 2336.
|
| [17] |
Titcomb, L. R.; Caddick, S.; Cloke, F. G. N.; Wilson, D. J.; Mckerrecher, D. Chem. Commun. 2001, 15, 1388.
|
| [18] |
Marion, N.; Navarro, O.; Mei, J.; Stevens, E. D.; Scott, N. M.; Nolan, S. P. J. Am. Chem. Soc. 2006, 128, 4101.
doi: 10.1021/ja057704z |
| [19] |
Schneider, S. K.; Herrmann, W. A.; Herdtweck, E. J. Mol. Catal. A: Chem. 2006, 245, 248.
doi: 10.1016/j.molcata.2005.08.046 |
| [20] |
Viciu, M. S.; Germaneau, R. F.; Nolan, S. P. Org. Lett. 2002, 4, 4053.
doi: 10.1021/ol026745m |
| [21] |
Viciu, M. S.; Kelly, R. A.; Stevens, E. D.; Naud, F.; Studer, M.; Nolan, S. P. Org. Lett. 2003, 5, 1479.
doi: 10.1021/ol034264c |
| [22] |
Marion, N.; Navarro, O.; Mei, J.; Stevens, E. D.; Scott, N. M.; Nolan, S. P. J. Am. Chem. Soc. 2006, 128, 4101.
doi: 10.1021/ja057704z |
| [23] |
Jackstell, R.; Andreu, M. G.; Frisch, A.; Selvakumar, K.; Zapf, A.; Klein, H.; Spannenberg, A.; Röttger, D.; Briel, O.; Karch, R.; Beller, M. Angew. Chem., Int. Ed. 2002, 41, 986.
|
| [24] |
O’Brien, C. J.; Kantchev, E. A. B.; Valente, C.; Hadei, N.; Chass, G. A.; Lough, A.; A. Hopkinson, C.; Organ, M. G. Chem.-Eur. J. 2006, 12, 4743.
doi: 10.1002/chem.200600251 |
| [25] |
Kantchev, E. A.; O’Brien, C. J.; Organ, M. G. Angew. Chem., Int. Ed. 2007, 46, 2768.
doi: 10.1002/anie.200601663 |
| [26] |
Organ, M. G.; Çlimsiz, S.; Sayah, M.; Hoi, K. H.; Lough, A. J. Angew. Chem., Int. Ed. 2009, 48, 2383.
doi: 10.1002/anie.200805661 |
| [27] |
Valente, C.; Calimsiz, S.; Hoi, K. H.; Mallik, D.; Sayah, M.; Organ, M. G. Angew. Chem., Int. Ed. 2012, 51, 3314.
doi: 10.1002/anie.201106131 |
| [28] |
Pompeo, M.; Froese, R. D. J.; Hadei, N.; Organ, M. G. Angew. Chem., Int. Ed. 2012, 51, 11354.
doi: 10.1002/anie.201205747 |
| [29] |
Tang, Y. Q.; Lu, J. M.; Shao, L. X. J. Organomet. Chem. 2011, 696, 3741.
doi: 10.1016/j.jorganchem.2011.08.042 |
| [30] |
Marion, N.; Nolan, S. P. Acc. Chem. Res. 2008, 41, 1440.
doi: 10.1021/ar800020y |
| [31] |
Nasielski, J.; Hadei, N.; Achonduh, G.; Kantchev, E. A. B.; O’Brien, C. J.; Lough, A.; Organ, M. G. Chem.-Eur. J. 2010, 16, 10844.
doi: 10.1002/chem.201000138 pmid: 20665575 |
| [32] |
Shen, A.; Ni, C.; Cao, Y. C.; Zhou, H.; Song, G. H. Tetrahedron Lett. 2014, 55, 3278.
doi: 10.1016/j.tetlet.2014.04.044 |
| [33] |
Shen, A.; Hu, Y. C.; Liu, T. T.; Ni, C.; Luo, Y.; Cao, Y. C. Tetrahedron Lett. 2016, 57, 2055.
doi: 10.1016/j.tetlet.2016.03.086 |
| [34] |
Hruszkewycz, D. P.; Balcells, D.; Guard, L. M.; Hazari, N.; Tilset, M. J. Am. Chem. Soc. 2014, 136, 7300.
doi: 10.1021/ja412565c pmid: 24824779 |
| [35] |
Kuwabe, S. I.; Torraca, K. E.; Buchwald, S. L. J. Am. Chem. Soc. 2001, 123, 12202.
pmid: 11734019 |
| [36] |
Zhang, L.; Yang, C.; Guo, X.-F.; Mo, F.-Y. Chin. J. Org. Chem. 2021, 41, 3492. (in Chinese)
doi: 10.6023/cjoc202103040 |
|
(张雷, 杨晨, 郭雪峰, 莫凡洋, 有机化学, 2021, 41, 3492.)
doi: 10.6023/cjoc202103040 |
|
| [37] |
Frisch, M. J.; Trucks, G. W.; Schlegel, H. B.; Scuseria, G. E.; Robb, M. A.; Cheeseman, J. R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G. A.; Nakatsuji, H.; Caricato, M.; Li, X.; Hratchian, H. P.; Izmaylov, A. F.; Bloino, J.; Zheng, G.; Sonnenberg, J. L.; Hada, M.; Ehara, M.; Toyota, K.; Fukuda, R.; Hasegawa, J.; Ishida, M.; Nakajima, T.; Honda, Y.; Kitao, O.; Nakai, H.; Vreven, T.; Montgomery, J. A. Jr.; Peralta, J. E.; Ogliaro, F.; Bearpark, M.; Heyd, J. J.; Brothers, E.; Kudin, K. N.; Staroverov, V. N.; Keith, T.; Kobayashi, R.; Normand, J.; Raghavachari, K.; Rendell, A.; Burant, J. C.; Iyengar, S. S.; Tomasi, J.; Cossi, M.; Rega, N.; Millam, J. M.; Klene, M.; Knox, J. E.; Cross, J. B.; Bakken, V.; Adamo, C.; Jaramillo, J.; Gomperts, R.; Stratmann, R. E.; Yazyev, O.; Austin, A. J.; Cammi, R.; Pomelli, C.; Ochterski, J. W.; Martin, R. L.; Morokuma, K.; Zakrzewski, V. G.; Voth, G. A.; Salvador, P.; Dannenberg, J. J.; Dapprich, S.; Daniels, A. D.; Farkas, O.; Foresman, J. B.; Ortiz, J. V.; Cioslowski, J.; Fox, D. J. Gaussian 09, Revision D.01, Gaussian, Inc., Wallingford CT, 2013.
|
| [38] |
Zhao, Y.; Truhlar, D. G. Acc. Chem. Res. 2008, 41, 157.
doi: 10.1021/ar700111a |
| [39] |
Marenich, A. V.; Cramer, C. J.; Truhlar, D. G. J. Phys. Chem. B 2009, 113, 6378.
doi: 10.1021/jp810292n |
| [1] | 刘悦灵, 钟欣欣, 张干兵. Pd(0)催化1-R-3-苯基亚丙基环丙烷(R=Me/H)与呋喃甲醛[3+2]环加成反应机理的密度泛函理论研究[J]. 有机化学, 2023, 43(2): 660-667. |
| [2] | 黄泽鑫, 尹宇强, 贾丰成, 吴安心. 吲哚及其衍生物C2—C3键断裂的反应研究进展[J]. 有机化学, 2022, 42(7): 2028-2044. |
| [3] | 石宇冰, 白文己, 母伟花, 李江平, 于嘉玮, 连冰. 钯催化C—H键官能团化形成C—X (X=O, N, F, I, ……)键的密度泛函理论研究进展[J]. 有机化学, 2022, 42(5): 1346-1374. |
| [4] | 朱有财, 丁欣欣, 孙莉, 刘振. CO2/C2H4耦合制备丙烯酸及其衍生物的研究进展[J]. 有机化学, 2022, 42(4): 965-977. |
| [5] | 李征, 谷迎春, 徐大振, 费学宁, 张磊. 有机膦催化的[4+2]环加成反应机理的密度泛函理论研究[J]. 有机化学, 2022, 42(3): 830-837. |
| [6] | 徐曼, 夏远志. 铑(III)催化N-苯氧基乙酰胺与亚甲基氧杂环丁酮氧化还原中性的碳氢活化/环化反应的机理研究[J]. 有机化学, 2021, 41(8): 3272-3278. |
| [7] | 高中润, 王媛, 宋航, 徐正仁, 贾彦兴. 吲哚苄位碳正离子引发的串联环化反应的普适性和机理探究[J]. 有机化学, 2021, 41(8): 3126-3133. |
| [8] | 杨凯, 刘美娟, 张毓娜, 占佳琦, 邓璐璇, 郑雪洁, 周永军, 汪朝阳. 基于2-卤苯甲酰胺合成苯并杂环化合物的研究进展[J]. 有机化学, 2021, 41(6): 2175-2187. |
| [9] | 贾丰成, 罗娜, 徐程, 吴安心. 靛红在苯并氮杂环类化合物的合成应用进展[J]. 有机化学, 2021, 41(4): 1527-1542. |
| [10] | 刘亮, 刘文波, 崔冬梅, 曾明. 芳酮类化合物的合成研究进展[J]. 有机化学, 2021, 41(11): 4289-4305. |
| [11] | 徐鑫明, 杨翰林, 李文忠. 无过渡金属的烯烃和芳烃C—H键的巯基化反应[J]. 有机化学, 2020, 40(7): 1912-1925. |
| [12] | 李志锋, 王文鹏, 王喜存, 权正军. 无酸条件下磷氰酸钠与胺反应合成磷代脲机理的密度泛函理论研究[J]. 有机化学, 2020, 40(6): 1563-1570. |
| [13] | 徐鑫明, 李家柱, 王祖利. 无过渡金属催化的吲哚C—H键的巯基化反应研究进展[J]. 有机化学, 2020, 40(4): 886-898. |
| [14] | 董道青, 孙媛媛, 李光辉, 杨欢, 王祖利, 徐鑫明. 喹啉氮氧化物的功能化反应研究进展[J]. 有机化学, 2020, 40(12): 4071-4086. |
| [15] | 陈晶晶, 王莹淑, 余珺, 成佳佳, 郑辉东. 钴(II)催化2-乙基-3-甲基吡嗪绿色氧化方法[J]. 有机化学, 2020, 40(1): 78-83. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||