有机化学 ›› 2013, Vol. 33 ›› Issue (02): 312-318.DOI: 10.6023/cjoc201210051 上一篇 下一篇
研究论文
姚春所, 林茂, 杨庆云
Yao Chunsuo, Lin Mao, Yang Qingyun
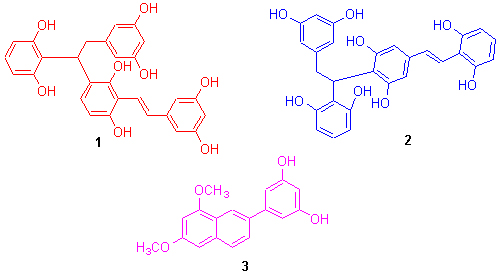
以天然得到的买麻藤醇为原料, 以FeCl3·6H2O为氧化剂进行氧化偶联反应和酸催化二聚反应, 获得了2个新的买麻藤醇二聚体及一个新的苯基萘衍生物: 4-[1-(2,6-二羟基苯基)-2-(3,5-二羟基苯基)乙基]-2-[(1E)-2-(3,5-二羟基苯基)乙烯基]-1,3-苯二醇(1), 2-[1-(2,6-二羟基苯基)-2-(3,5-二羟基苯基)乙基]-5-[(1E)-2-(2,6-二羟基苯基)乙烯基]-1,3-苯二醇(2)和4-(6,8-二甲氧基-2-萘基)-1,3-苯二醇(3). 应用波谱分析的方法确定了它们的结构, 并分别讨论了它们可能的形成机理. 其中, 化合物1和2首次为人工合成的二苯乙烯链状二聚体. 活性测试结果表明, 化合物1, 2和3显示有较强的抗氧化活性, 其IC50值分别为6.29×10-9, 4.19×10-6和2.96×10-5 mol·L-1; 化合物2还显示有较强的抗炎活性.