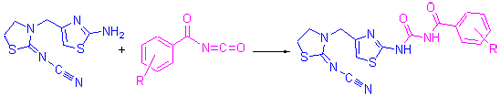
| [1] Zhao, P. L.; Wang, F.; Huang, W.; Chen, Q.; Liu, Z. M. Chin. J. Org. Chem. 2010, 30, 1567 (in Chinese). (赵培亮, 王福, 黄伟, 陈琼, 刘祖明, 有机化学, 2010, 30, 1567.)[2] Zhao, J. H.; Zhou, Y.; Xu, X. H.; Zhu, L.; Zhu, G. N.; Cheng, J. L. Chin. J. Org. Chem. 2010, 30, 719 (in Chinese). (赵金浩, 周勇, 徐旭辉, 朱烈, 朱国念, 程敬丽, 有机化学, 2010, 30, 719.)[3] Takano, M.; Enomoto, M.; Saito, K.; Kizawa, S. EP 683160, 1995[Chem. Abstr. 1995, 124, 202283].[4] Hong, Y. P.; Song, B. A.; Wu, P.; Yan, X. Z.; Liu, N. J. Anhui Agric. Sci. 2005, 33, 1254 (in Chinese). (洪艳平, 宋宝安, 吴平, 颜贤仔, 刘楠, 安徽农业科学, 2005, 33, 1254.)[5] Zhang, H.; Li, X. Y.; Hu, D. Y.; Yang, S.; Fan, H. T.; Wei, X.; Song, B. A. Chin. J. Org. Chem. 2011, 31, 1419 (in Chinese). (章浩, 李向阳, 胡德禹, 杨松, 范会涛, 魏学, 宋宝安, 有机化学, 2011, 31, 1419.)[6] O'Reilly, P.; Kobayashi, S.; Yamane, S.; Phillips, W. G.; Raymond, P.; Castanho, B. In Proceedings of the Brighton Crop Protection Conferences, Pest and Diseases, British Crop Protection Council, Brighton, 1992, p. 427.[7] Kim, D. S.; Chun, S. J.; Jeon, J. J.; Lee, S. W.; Joe, G. H. Pest Manage. Sci. 2004, 60, 1007.[8] Maienfisch, P. L.; Huerlimann, H.; Rindlisbacher, A.; Gsell, L.; Dettwiler, H.; Haettenschwiler, J.; Sieger, E.; Walti, M. Pest Manage. Sci. 2001, 57, 165.[9] Dai, B. J. World Pestic. 2005, 27, 46 (in Chinese). (戴宝江, 世界农药, 2005, 27, 46.)[10] Li, N.; Wei, X.; Fan, H. T.; Bi, L.; Li, X. Y.; Yang, S.; Hu, D. Y.; Song, B. A. Chin. J. Org. Chem. 2011, 31, 1300 (in Chinese). (李娜, 魏学, 范会涛, 毕亮, 李向阳, 杨松, 胡德禹, 宋宝安, 有机化学, 2011, 31, 1300.)[11] Ling, Y.; Zhu, W. W.; Shi, W. P.; Li, X. C.; Qiu, L. H.; Sun, Y. F.; Yang, X. L. Chin. J. Pestic. Sci. 2008, 10, 141 (in Chinese). (凌云, 朱伟文, 石旺鹏, 李希晨, 邱立红, 孙玉凤, 杨新玲, 农药学学报, 2008, 10, 141.)[12] Jiang, L.; Liu, F.; Zhang, D. K.; Wang, H. B. J. Pestic. Sci. 2010, 35, 33.[13] Xue, S. J.; Ke, S. Y.; Duan, L. P. Chin. J. Org. Chem. 2004, 24, 227 (in Chinese). (薛思佳, 柯少勇, 段李平, 有机化学, 2004, 24, 227.)[14] Song, X. J.; Tang, X. H.; Yu, A. N.; Wang, Y. G. Chin. J. Org. Chem. 2006, 26, 803 (in Chinese). (宋新建, 谭小红, 余爱农, 汪焱钢, 有机化学, 2006, 26, 803.)[15] Song, X. J.; Wang, S.; Tan, X. H.; Wang, Z. Y.; Wang, Y. G. Chin. J. Org. Chem. 2007, 27, 72 (in Chinese). (宋新建, 王胜, 谭小红, 王子云, 汪焱钢, 有机化学, 2007, 27, 72.)[16] Sun, C. W.; Zhang, X. D.; Huang, H.; Zhou, P. Bioorg. Med. Chem. 2006, 14, 8574.[17] Dai, H.; Miao, W. K.; Liu, J. B.; Wu, S. S.; Qin, X.; Zhang, X.; Fang, J. X. Chin. J. Org. Chem. 2012, 32, 1327 (in Chinese). (戴红, 苗文科, 刘建兵, 吴珊珊, 秦雪, 张欣, 方建新, 有机化学, 2012, 32, 1327.)[18] Hanson, R. N.; Davis, M. A. J. Heterocycl. Chem. 1981, 18, 205.[19] Dai, H.; Liu, J. B.; Zhang, X.; Yu, H. B.; Qin, X.; Qin, Z. F.; Wang, T. T.; Fang, J. X. Chin. J. Org. Chem. 2009, 29, 123 (in Chinese). (戴红, 刘建兵, 张欣, 于海波, 秦雪, 秦振芳, 王婷婷, 方建新, 有机化学, 2009, 29, 123.)[20] Liu, J. B.; Tao, W. F.; Hu, Y.; Dai, H.; Fang, J. X. Chin. J. Org. Chem. 2006, 26, 1566 (in Chinese). (刘建兵, 陶伟峰, 胡燕, 戴红, 方建新, 有机化学, 2006, 26, 1566.) |