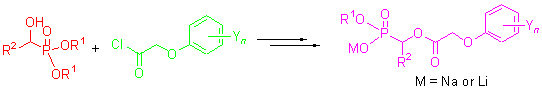| [1] Baillie, A. C.; Wright, K.; Wright, B. J.; Earnshaw, C. G. Pestic. Biochem. Physiol. 1988, 30, 103.[2] Kluger, R.; Pike, D. C. J. Am. Chem. Soc. 1977, 99, 4504.[3] Kluger, R.; Gish, G.; Kauffman, G. J. Biol. Chem. 1984, 259, 8960.[4] He, H. W.; Yuan, J. L. Peng, H.; Chen, T.; Shen, P.; Wan, S. Q.; Li, Y. J.; Tan, H. L.; He, Y. H.; He, J. B.; Li, Y. J. Agric. Food Chem. 2011, 59, 4801.[5] Wang, T.; He, H. W.; Miao, F. M. Chin. J. Org. Chem. 2009, 29, 1152 (in Chinese).(贺红武, 刘建超, 缪方明, 有机化学, 2009, 29, 1152.)[6] He, H. W.; Wang, T.; Yuan, J. L. J. Organomet. Chem. 2005, 690, 2608.[7] Peng, H.; Wang, T.; Xie, P.; Chen, T.; He, H. W.; Wang, J. J. Agric. Food Chem. 2007, 55, 1871.[8] Wang, T.; He, H. W. Synth. Commun. 2004, 34, 1415.[9] He, H. W.; Peng, H.; Wang, T.; et al.Wang, C. B.; Yuan, J. L.; Chen, T.; He, J. B.; Tan, X. S., J. Agric. Food Chem. 2013, 61, 2479.[10] Texier-Boullet, F.; Foucaud, A. Synthesis 1982, 916.[11] Peng, H.; He, H. W. Chin. J. Org. Chem. 2007, 27, 502 (in Chinese).(彭浩, 贺红武, 有机化学, 2007, 27, 502.)[12] Liu, J. C.; Cui, Z. P.; He, H. W. Chin. J. Org. Chem. 2011, 31, 2082 (in Chinese).(刘建超, 崔泽平, 贺红武, 有机化学, 2011, 31, 2082.)[13] .Lei, J. P.; Han, J. T.; X, Z. H.; Dong, H. B.; Wang, M. A. Chin. J. Org. Chem. 2012, 32, 1993 (in Chinese).(雷建平, 韩金涛, 徐志红, 董宏波, 王明安, 有机化学, 2012, 32, 1993.) |