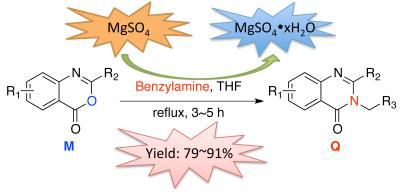
| [1] Chen, H.-J.; Jiang, Y.-L.; Lin, C.-M. Int. J. Oncol. 2013, 43, 141. [2] Patel, M. B.; Kumar, S. P.; Valand, N. N. J. Mol. Model. 2013, 19, 3201. [3] Chao, Q.; Deng, L.; Shih, H. J. Med. Chem. 1999, 42, 3860. [4] Beaulieu, P. L.; Coulombe, R.; Duan, J. Bioorg. Med. Chem. Lett. 2013, 23, 4132. [5] Sahoo, B. M.; Dinda, S. C.; Kumar, B. V. V. R. Int. J. Pharm. Sci. Nanotechnol. 2013, 6, 2046. [6] Abou-Seri, S. M.; Abouzid, K.; Abou El Ella, D. A. Eur. J. Med. Chem. 2011, 46, 647. [7] Antonelli, A.; Fallahi, P.; Ferrari, S. M. Curr. Genomics 2011, 12, 626. [8] Morgensztern, D.; Govindan, R. Chemother. Source Book, 4th ed., 2008, p. 191. [9] (a) Zhou, J.; Fang, J. J. Org. Chem. 2011, 76, 7730.(b) Sim, Y.-L.; Omer, N.; Khan, M. N. Tetrahedron 2013, 69, 2524.(c) Chen, Y.; Shan, W.; Lei, M. Tetrahedron Lett. 2012, 53, 5923.(d) Badri, R.; Alizadeh-Haddad, A.; Adlu, M. Bull. Chem. Soc. Ethiop. 2013, 27, 131. [10] (a) Gao, X.; Cai, X.; Yan, K. Chin. J. Org. Chem. 2008, 28, 1785 (in Chinese).(高兴文, 蔡学建, 严凯, 有机化学, 2008, 28, 1785.;b) Gao, X.; Cai, X.; Yan, K. Molecules 2007, 12, 2621.[11] Xie, M.; Chen, G.; Zou, L.Chem. Res. Appl. 2010, 864 (in Chinese).(谢敏, 陈广民, 邹丽娟, 化学研究与应用, 2010, 864.)[12] (a) Wang, X.; Li, P.; Li, Z. J. Agric. Food Chem. 2013, 61, 9575.(b) Nanda, A. K.; Ganguli, S.; Chakraborty, R. Molecules 2007, 12, 2413.(c) Ouyang, G.; Zhang, P.; Xu, G. Molecules 2006, 11, 383. [13] (a) Liu, J.-F. Curr. Org. Synth. 2007, 4, 223.(b) Besson, T.; Chosson, E. Comb. Chem. High Throughput Screening 2007, 10, 903.(c) El-Mekabaty, A. Int. J. Mod. Org. Chem. 2013, 2, 81. [14] Patil, D. A.; Patil, P. O.; Patil, G. B. Mini-Rev. Med. Chem. 2011, 11, 633. [15] (a) Salehi, P.; Dabiri, M.; Zolfigol, M. A. Tetrahedron Lett. 2005, 41, 7051.(b) Baghbanzadeh, M.; Dabiri, M.; Salehi, P. Heterocycles 2008, 11, 2809.(c) Chen, J.; Wu, D.; He, F. Tetrahedron Lett. 2008, 23, 3814.(d) Ishikawa, K.; Hosoe, T.; Itabashi, T. Sci. Pharm. 2011, 79, 937. [16] O'Neil, M. J. The Merck Index: An Encyclopedia of Chemicals, Drugs, and Biologicals, 14th ed., Merck & Co., Inc., New Jersey, 2006, Monograph Number: 1658, 2653, 5691 and 8680. [17] (a) Sati, N.; Kumar, S.; Rawat, M. S. M. Indian J. Pharm. Sci. 2009, 71, 572.(b) Tseng, M.-C.; Chu, Y.-H. Tetrahedron 2008, 64, 9515. [18] (a) Nerkar, A. G.; Kudale, S. A.; Joshi, P. P. Int. J. Pharm. Pharm. Sci. 2012, 4, 449.(b) Chikhale, H.; Lade, K.; Joshi, P. Int. J. Pharm. Pharm. Sci. 2012, 4, 466. [19] Armarego, W. L. F.; Chai, C. L. L. Purification of Laboratory Chemicals, 5th ed., Butterworth Heinemann Press, London, 2003, p. 361, p. 378. [20] Muller, P.; Herbst, I. R.; Spek, A. L.; Schneider, T. R.; Sawaya, M. R. Crystal Structure Refinement—A Crystallographer's Guide to SHELXL, Oxford University Press, New York, 2006. |