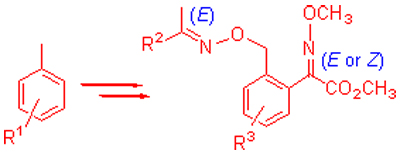
| [1] Beautement, K.; Clough, J. M.; de Fraine, P. J.; Godfrey, C. R. A. Pestic. Sci. 1991, 31, 499.[2] Sauter, H.; Steglich, W.; Anke, T. Angew. Chem., Int. Ed. 1999, 38, 1329.[3] Lu, C.-Z.; Liu, J.-H.; Du, X.-H. Agrochemicals 2011, 50, 187 (in Chinese).(陆翠军, 刘建华, 杜晓华, 农药, 2011, 50, 187.)[4] Zhao, P. Pestic. Alerts 2012, 25 (in Chinese).(赵平, 农药快讯, 2012, 25.)[5] Li, M.; Liu, C.-L.; Yang, J.-C.; Zhang, J.-B.; Li, Z.-N.; Zhang, H.; Li, Z.-M. J. Agric. Food Chem. 2010, 58, 2664.[6] Hua, N.-Z. Modern Agrochem. 2013, 12, 6 (in Chinese).(华乃震, 现代农药, 2013, 12, 6.)[7] Sun, R.-F.; Lu, M..-Y.; Chen, L.; Li, Q.-S.; Song, H.-B.; Bi, F.-C.; Huang, R.-Q.; Wang, Q.-M. J. Agric. Food Chem. 2008, 56, 11376[8] Zhang, X.; Gao, Y.-X.; Liu, H.-J. Bull. Korean Chem. Soc. 2012, 33, 2627.[9] Chai, B.; Wen, C.; Chen, X.-D.; Li, Z.-C. Agrochemicals 2013, 52, 258.(柴兵, 闻冲, 陈晓东, 李宗成, 农药, 2013, 52, 258.)[10] Chung, W. H.; Deepak, S. A.; Ishii, H. J. Gen. Plant Pathol. 2013, 79, 128.[11] Huang, W.; Zhao, P.-L.; Liu, C.-L.; Chen, Q.; Liu, Z.-M.; Yang, G.-F. J. Agric. Food Chem. 2007, 55, 3004.[12] Zhao, P.-L.; Zhang, B.; Wang, Y.-Z.; Yang, G.-F. Chin. J. Org. Chem. 2008, 28, 875 (in Chinese).(赵培亮, 章博, 王亚洲, 杨光富, 有机化学, 2008, 28, 875.)[13] Bostanian, N. J.; Larocque, N. Phytoparasitica 2011, 29, 215[14] Li, Q. M.S. Thesis, Tongji University, Shanghai, 2007 (in Chinese).(李倩, 硕士论文, 同济大学, 上海, 2007).[15] Wang, X.-Y.; Xue, X.-P.; Pang, Y.-P.; Chen, P.-Y.; Min, N.-N.; Dou, Y.-L. J. Jilin Agric. Sci. 2013, 38, 49 (in Chinese).(王献友, 薛潇沛, 庞艳萍, 陈培云, 闵娜娜, 窦玉蕾, 吉林农业科学, 2013, 38, 49.)[16] Zhao, P.; Yan, Q.-X; Li, X.; Zhang, M.-H. Agrochemicals 2011, 50, 547 (in Chinese).(赵平, 严秋旭, 李新, 张敏恒, 农药, 2011, 50, 547.)[17] Liu, C.-L.; Zhang, L.-X.; Wang, C.-M.; She, Y.-H. Pesticides 1998, 37, 1 (in Chinese).(刘长令, 张立新, 汪灿明, 佘永红, 农药, 1998, 37, 1.)[18] Liu, A.-P.; He, H.-J.; Liu, X.-P.; He, L.; Hu, C.-H.; Wei, Z.-Z.; Hu, Z.-B.; Hang, M.-Z.; Ou, X.-M.; Su, S.-P. Chin. J. Org. Chem. 2009, 29, 1381 (in Chinese).(柳爱平, 何海军, 刘兴平, 何莲, 胡春华, 魏振中, 胡志彬, 黄明智, 欧晓明, 苏胜培, 有机化学, 2009, 29, 1381.)[19] Pascual, A.; Ziegler, H.; Trah, S.; Ertl, P.; Winkler, T. Tetrahedron Lett. 2000, 41, 1381.[20] Kuroda, Y.; Yamada, M.; Tabushi, I. J. Chem. Soc., Perkin Trans. 2 1989, 1409.[21] Chen, C.; Liu, A.-P.; Mao, C.-H.; Liu, X.-P. Fine Chem. Intermed. 2004, 34, 25 (in Chinese).(陈灿, 柳爱萍, 毛春辉, 刘兴平, 精细化工中间体, 2004, 34, 25.)[22] Kikuchi, D.; Sakaguchi, S.; Ishii, Y. J. Org. Chem. 1998, 63, 6023.[23] Jiri, H.; Illia, P.; Milos, S. Tetrahedron Lett. 2013, 54, 3533.[24] Judd, L. W.; Davis, A. P. Chem. Commun. 2010, 46, 2227.[25] Xu, P.-P. M.S. Thesis, Huazhong Normal University, Hubei, 2012 (in Chinese).(徐平平, 硕士论文, 华中师范大学, 湖北, 2012.)[26] Zhang, X.; Zhang, Y.-J.; Chen, Y.; Zhou, M.-G. Chin. J. Pestic. Sci. 2008, 10, 41 (in Chinese).(张晓, 张艳军, 陈雨, 周明国, 农药学学报, 2008, 10, 41.) |