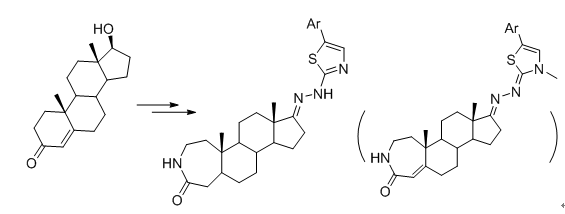
| [1] Festi, D.; Montagnani, M.; Azzaroli, F.; Lodato, F.; Mazzella, G.; Roda, A.; Di-Biase, A. R; Roda, E.; Simoni, P.; Colecchia, A. Curr.Clin.Pharmacol. 2007, 2, 155.
[2] Ifere, G.; Barr, E.; Equan, A.; Gordon, K.; Singh, U. P.; Chaudhary, J.; Igietseme, J. U.; Ananaba, G. A. Cancer Detect. Prev. 2009, 32, 319.
[3] Morzycki, J. W. Steroids 2014, 83, 62.
[4] Joao, F. S.; Carvalho, M.; Manuel, C. S.; Joao, N.; Moreira, S. S.; Melo, M. L. S. J.Med. Chem. 2011, 54, 6375.
[5] Jorge, A. R.; Salvador, J. F. S.; Carvalho, Marco A. C.; Neves, S. M.; Silvestre, A. J.; Leitao, M.; Manuel C. S. Nat. Prod.Rep. 2013, 30, 324.
[6] Ranju, B.; Pratap, C. A. Chem. Rev. 2014, 114, 6986.
[7] Liun, Q.-F.; Cui, J.-G.; Gan, C.-F. Chin. J.Org.Chem. 2012, 32, 2214(in Chinese). (林启福, 崔建国, 甘春芳, 刘亮, 姚秋翠, 黄燕敏, 有机化学, 2012, 32, 2214.)
[8] Huang, Y. M.; Cui, J. G.; Zhong, Z. G.; Gan, C. F.; Zhang, W. Y.; Song, H. C. Bioorg.Med.Chem.Lett. 2011, 21, 3641.
[9] Huang, Y. M.; Cui, J. G.; Chen, S. J.; Gan, C. F.; Zhou, A. M. Steroids 2011, 76, 1346.
[10] Huang, Y. M.; Cui, J. G.; Chen, S. J.; Lin, Q. F.; Song, H. C.; Gan, C.-F.; Su, B.; Zhou, A. M. Mar.Drugs 2014, 12, 1715.
[11] Huang, Y. M.; Cui, J. G.; Chen, S. J.; Gan, C.-F.; Yao, Q. C.; Lin, Q. F. Bioorg.Med.Chem.Lett. 2013, 23, 2265.
[12] Cui, S. F.; Wang, Y.; Lv, J. S.; Damu Guri L. V.; Zhou C. H. Sci. China Chem. 2012, 42, 1105(in Chinese). (崔胜峰, 王艳, 吕敬松, Damu Guri L. V., 周成合, 中国科学:化学, 2012, 42, 1105.)
[13] Kashfi, K. Adv. Pharmacol. 2009, 57, 31.
[14] Sun, L. P.; Jiang, Z.; Gao, L. X.; Liu, X. F.; Quan, Y. C.; Zheng, G. H.; Li, J.; Piao, H. Chin. J. Org. Chem. 2013, 33, 1496.
[15] Mohareb, R. M.; Al-Omran, F. Steroids 2012, 77, 1551.
[16] Cui, J. G.; Liu, L.; Gan, C. F.; Xiao, Q.; Huang, Y. M. Prog. Chem. 2014, 26, 320(in Chinese). (崔建国, 刘亮, 甘春芳, 肖琦, 黄燕敏, 化学进展, 2014, 26, 320.)
[17] Cui, J. G.; Zhao, D. D.; He, D. M.; Huang, Y. M.; Liu, Z. P.; Liu, X. F.; Shi, H. X.; Gan, C. F. Chin. J. Org. Chem. 2016, 36, 630(in Chinese). (崔建国, 赵丹丹, 何冬梅, 黄燕敏, 刘志平, 林啟福, 石海信, 甘春芳, 有机化学, 2016, 36, 630.)
[18] Huang, Y. M.; Qi, B. B.; Cui, J. G.; Gan, C. F.; Yang, C. H.; Liu, C.; Chen, S.; Shi, H. X. Chin. J. Org. Chem. 2016, 36, 1602(in Chinese). (黄燕敏, 戚斌斌, 崔建国, 甘春芳, 杨春晖, 刘畅, 陈爽, 石海信, 有机化学, 2016, 36, 1602.) |