有机化学 ›› 2023, Vol. 43 ›› Issue (6): 2126-2135.DOI: 10.6023/cjoc202209040 上一篇 下一篇
研究论文
庞盼杏a, 宁蓉a, 祝创a, 黄文洁a, 马献力a, 蒋彩娜a, 李芳耀a,*( ), 周小群b,*(
), 周小群b,*( )
)
收稿日期:2022-09-30
修回日期:2022-12-11
发布日期:2023-01-11
作者简介:基金资助:
Panxing Panga, Rong Ninga, Chuang Zhua, Wenjie Huanga, Xianli Maa, Caina Jianga, Fangyao Lia,*( ), Xiaoqun Zhoub,*(
), Xiaoqun Zhoub,*( )
)
Received:2022-09-30
Revised:2022-12-11
Published:2023-01-11
Contact:
E-mail: About author:Supported by:文章分享

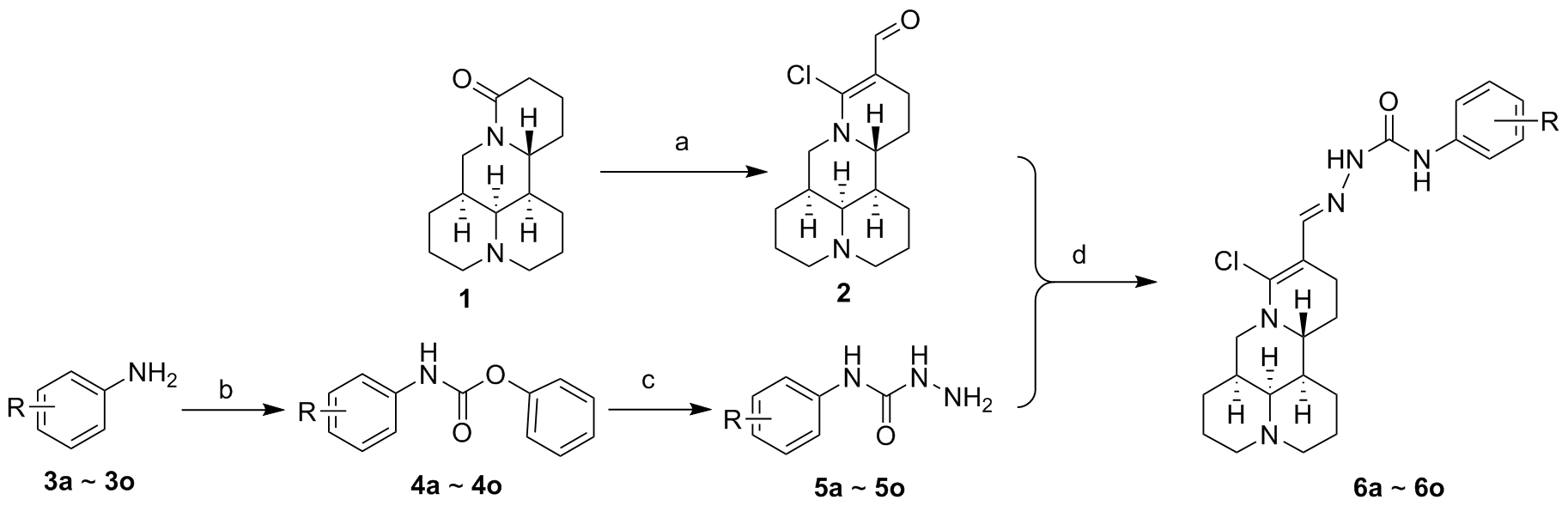
为了寻找高效低毒的新型抗肿瘤化合物, 设计并合成了一系列新型的苦参碱缩氨基脲类化合物. 采用噻唑蓝(MTT)法测定目标化合物对人肺癌细胞(A549)、人肝癌细胞(HepG2)、人宫颈癌细胞(Hela)、人胃癌细胞(MGC803)细胞株及人正常肝细胞株(L-O2)的细胞毒活性. 结果表明, 大多数衍生物对肿瘤细胞的细胞毒性明显高于母体化合物. 其中14-甲酰基-15-氯-苦参碱缩N-(3-氯苯基)氨基脲(6c)和14-甲酰基-15-氯-苦参碱缩N-(3-硝基苯基)氨基脲(6f)对人肺癌A549细胞株表现出良好的抑制活性, IC50值分别为(11.70±0.25)和(15.61±0.07) μmol/L, 活性优于阳性对照药喜树碱. 同时, 对人胃癌MGC803细胞株IC50值分别为(11.32±1.07)和(9.27±2.03) μmol/L, 并且对人正常肝细胞株(L-O2)表现出较低的细胞毒性. 流式细胞术及线粒体膜电位检测表明, 化合物6f能够剂量依赖性地诱导MGC803细胞凋亡, 并降低线粒体膜电位. 上述研究为苦参碱的结构修饰提供了思路.
庞盼杏, 宁蓉, 祝创, 黄文洁, 马献力, 蒋彩娜, 李芳耀, 周小群. 苦参碱缩氨基脲类化合物的合成及其体外抗肿瘤活性研究[J]. 有机化学, 2023, 43(6): 2126-2135.
Panxing Pang, Rong Ning, Chuang Zhu, Wenjie Huang, Xianli Ma, Caina Jiang, Fangyao Li, Xiaoqun Zhou. Synthesis and in Vitro Antitumor Activity of Matrine Semicarbazide Derivatives[J]. Chinese Journal of Organic Chemistry, 2023, 43(6): 2126-2135.

| Complex | 6a |
|---|---|
| Empirical formula | C23H28ClN5O |
| Formula weight (Mr) | 425.95 |
| Crystal system | Orthorhombic |
| Space group | P212121 |
| a/nm | 0.52876(1) |
| b/nm | 1.53224(3) |
| c/nm | 2.68038(5) |
| V/nm3 | 2.17161(7) |
| Z | 4 |
| F(000) | 904 |
| Dx/(Mg•m–3) | 1.303 |
| Cu Kα radiation (λ)/nm | 0.154184 |
| μ/mm–1 | 1.75 |
| T/K | 293 |
| Measured reflections | 16468 |
| Independent reflections | 4367 |
| Reflections with I>2(I) | 3707 |
| Rint | 0.052 |
| Complex | 6a |
|---|---|
| Empirical formula | C23H28ClN5O |
| Formula weight (Mr) | 425.95 |
| Crystal system | Orthorhombic |
| Space group | P212121 |
| a/nm | 0.52876(1) |
| b/nm | 1.53224(3) |
| c/nm | 2.68038(5) |
| V/nm3 | 2.17161(7) |
| Z | 4 |
| F(000) | 904 |
| Dx/(Mg•m–3) | 1.303 |
| Cu Kα radiation (λ)/nm | 0.154184 |
| μ/mm–1 | 1.75 |
| T/K | 293 |
| Measured reflections | 16468 |
| Independent reflections | 4367 |
| Reflections with I>2(I) | 3707 |
| Rint | 0.052 |
| Compd. | R | IC50a/(μmol•L–1) | ||||
|---|---|---|---|---|---|---|
| L-O2 | MGC803 | HeLa | A549 | Hepg2 | ||
| 6a | H | 30.46±0.12 | 17.41±1.52 | 24.86±0.70 | 46.53±0.40 | 83.93±1.30 |
| 6b | 2-Cl | 76.50±2.82 | 11.66±1.31 | 17.30±2.04 | 25.48±2.72 | >100 |
| 6c | 3-Cl | 27.45±2 47 | 11.32±1.07 | 16.94±1.97 | 11.70±0.25 | 35.82±1.93 |
| 6d | 4-Cl | 12.77±1.87 | 14.36±2.99 | 15.47±1.24 | 45.48±1.11 | 46.75±1.59 |
| 6e | 2-NO2 | 31.46±0.98 | 17.47±2.58 | 32.51±0.14 | 33.31±0.03 | >100 |
| 6f | 3-NO2 | 63.99±2.71 | 9.27±2.03 | 50.84±0.91 | 15.61±0.07 | 27.59±2.02 |
| 6g | 4-NO2 | 33.24±0.74 | 21.70±1.89 | 30.21±1.71 | 63.74±1.21 | >100 |
| 6h | 2-Br | 45.85±1.47 | 23.63±0.22 | 38.34±0.65 | 71.39±4.84 | 59.82±2.85 |
| 6i | 4-Br | 12.36±2.64 | 13.73±1.57 | 16.34±1.87 | 20.72±0.49 | >100 |
| 6j | 2-F | 32.90±0.36 | 14.16±2.35 | 30.09±2.69 | 49.90±1.70 | >100 |
| 6k | 3-F | 26.70±1.64 | 16.46±1.99 | 23.50±2.8 | 62.73±2.42 | 38.64±4.47 |
| 6l | 4-F | 11.94±1.85 | 17.28±1.27 | 15.94±1.41 | 41.49±1.46 | 31.40±2.31 |
| 6m | 2-CH3 | 27.49±2.22 | 20.84±0.11 | 21.34±2.36 | 56.86±1.57 | 56.90±2.43 |
| 6n | 3-CH3 | 33.48±2.23 | 19.73±1.04 | 25.35±0.19 | 45.11±1.32 | 53.94±2.67 |
| 6o | 4-CH3 | 18.02±2.13 | 13.81±0.23 | 21.21±0.60 | 53.44±2.18 | 92.76±3.05 |
| 苦参碱 | >100 | >100 | >100 | >100 | >100 | |
| 喜树碱b | 4.44±2.54 | 8.13±2.13 | 2.00±0.8 | 20.34±1.53 | 2.30±1.19 | |
| Compd. | R | IC50a/(μmol•L–1) | ||||
|---|---|---|---|---|---|---|
| L-O2 | MGC803 | HeLa | A549 | Hepg2 | ||
| 6a | H | 30.46±0.12 | 17.41±1.52 | 24.86±0.70 | 46.53±0.40 | 83.93±1.30 |
| 6b | 2-Cl | 76.50±2.82 | 11.66±1.31 | 17.30±2.04 | 25.48±2.72 | >100 |
| 6c | 3-Cl | 27.45±2 47 | 11.32±1.07 | 16.94±1.97 | 11.70±0.25 | 35.82±1.93 |
| 6d | 4-Cl | 12.77±1.87 | 14.36±2.99 | 15.47±1.24 | 45.48±1.11 | 46.75±1.59 |
| 6e | 2-NO2 | 31.46±0.98 | 17.47±2.58 | 32.51±0.14 | 33.31±0.03 | >100 |
| 6f | 3-NO2 | 63.99±2.71 | 9.27±2.03 | 50.84±0.91 | 15.61±0.07 | 27.59±2.02 |
| 6g | 4-NO2 | 33.24±0.74 | 21.70±1.89 | 30.21±1.71 | 63.74±1.21 | >100 |
| 6h | 2-Br | 45.85±1.47 | 23.63±0.22 | 38.34±0.65 | 71.39±4.84 | 59.82±2.85 |
| 6i | 4-Br | 12.36±2.64 | 13.73±1.57 | 16.34±1.87 | 20.72±0.49 | >100 |
| 6j | 2-F | 32.90±0.36 | 14.16±2.35 | 30.09±2.69 | 49.90±1.70 | >100 |
| 6k | 3-F | 26.70±1.64 | 16.46±1.99 | 23.50±2.8 | 62.73±2.42 | 38.64±4.47 |
| 6l | 4-F | 11.94±1.85 | 17.28±1.27 | 15.94±1.41 | 41.49±1.46 | 31.40±2.31 |
| 6m | 2-CH3 | 27.49±2.22 | 20.84±0.11 | 21.34±2.36 | 56.86±1.57 | 56.90±2.43 |
| 6n | 3-CH3 | 33.48±2.23 | 19.73±1.04 | 25.35±0.19 | 45.11±1.32 | 53.94±2.67 |
| 6o | 4-CH3 | 18.02±2.13 | 13.81±0.23 | 21.21±0.60 | 53.44±2.18 | 92.76±3.05 |
| 苦参碱 | >100 | >100 | >100 | >100 | >100 | |
| 喜树碱b | 4.44±2.54 | 8.13±2.13 | 2.00±0.8 | 20.34±1.53 | 2.30±1.19 | |




| [1] |
Bray, F.; Laversanne, M.; Weiderpass, E.; Soerjomataram, I. Cancer 2021, 127, 3029.
doi: 10.1002/cncr.v127.16 |
| [2] |
Sung, H.; Ferlay, J.; Siegel, R. L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Ca-Cancer J. Clin. 2021, 71, 209.
doi: 10.3322/caac.v71.3 |
| [3] |
Alam, O.; Mullick, P.; Verma, S. P.; Gilani, S. J.; Khan, S. A.; Siddiqui, N.; Ahsan, W. Eur. J. Med. Chem. 2010, 45, 2467.
doi: 10.1016/j.ejmech.2010.02.031 |
| [4] |
Sriram, D.; Stables, P. J.; Thirumurugan, R.; Induja, S.; Ragavendran, V. J.; Yogeeswari, P. Med. Chem. 2006, 2, 55.
pmid: 16787356 |
| [5] |
Yogeeswari, P.; Sriram, D.; Veena, V.; Kavya, R.; Rakhra, K.; Ragavendran, J. V.; Mehta, S.; Thirumurugan, R.; Stables, J. P. Biomed. Pharmacother. 2005, 59, 51.
pmid: 15740936 |
| [6] |
Xu, H.; Su, X.; Liu, X.Q.; Zhang, K. P.; Hou, Z.; Guo, C. Bioorg. Med. Chem. Lett. 2019, 29, 23.
|
| [7] |
Hania, M. M. E-J. Chem. 2009, 6, 508.
|
| [8] |
Gopi, C.; Dhanaraju, D. M. Beni-Suef Univ. J. Basic Appl. Sci. 2018, 7, 291.
|
| [9] |
Chipeleme, A.; Gut, J.; Rosenthal, P. J.; Chibale, K. Bioorg. Med. Chem. 2007, 15, 273.
doi: 10.1016/j.bmc.2006.09.055 |
| [10] |
Queiroz, A. C.; Alves, M. A.; Barreiro, E. J.; Lima, L. M.; Alexandre-Moreira, M. S. Exp. Parasitol. 2019, 201, 57.
doi: 10.1016/j.exppara.2019.04.003 |
| [11] |
Qazi, S. U.; Naz, A.; Hameed, A.; Osra, F. A.; Jalil, S.; Iqbal, J.; Ali Shah, S. A.; Mirza, A. Z. Bioorg. Chem. 2021, 115, 105209.
doi: 10.1016/j.bioorg.2021.105209 |
| [12] |
Liu, Y.-B.; Peng, W.-L.; Yu, L.-J.; Xing, J.-H.; Chen, J.; Xia, X.-J.; Shen, D.-L. Agrochemicals 2010, 49, 407. (in Chinese)
|
|
(刘永榜, 彭伟立, 郁林军, 邢家华, 陈杰, 夏旭建, 沈德隆, 农药, 2010, 49, 407.)
|
|
| [13] |
Ma, J. J.; Hu, G.; Xie, L. J.; Chen, L.; Xu, B. X.; Gong, P. Chem. Res. Chin. Univ. 2015, 31, 958.
doi: 10.1007/s40242-015-5034-1 |
| [14] |
Jia, X. X.; Liu, Q.; Wang, S. Y.; Zeng, B. L.; Du, G. H.; Zhang, C.; Li, Y. Bioorg. Med. Chem. 2020, 28, 115557.
doi: 10.1016/j.bmc.2020.115557 |
| [15] |
Afrasiabi, Z.; Sinn, E.; Lin, W. S.; Ma, Y. F.; Campana, C.; Padhye, S. J. Inorg. Biochem. 2005, 99, 1526.
pmid: 15927263 |
| [16] |
Mishra, B. B.; Tiwari, V. K. Eur. J. Med. Chem., 2011, 46, 4769.
doi: 10.1016/j.ejmech.2011.07.057 |
| [17] |
Mondal, S.; Bandyopadhyay, S.; Ghosh, M. K.; Mukhopadhyay, S.; Roy, S.; Mandal, C. Anti-Cancer Agents Med. Chem. 2012, 12, 49.
doi: 10.2174/187152012798764697 |
| [18] |
Newman, D. J.; Cragg, G. M.; Snader, K. M. J. Nat. Prod. 2003, 66, 1022.
doi: 10.1021/np030096l pmid: 12880330 |
| [19] |
Huang, J. L.; Xu, H. Curr. Top. Med. Chem. 2016, 16, 3365.
doi: 10.2174/1568026616666160506131012 |
| [20] |
Pan, J. L.; Hao, X.; Yao, H. W.; Ge, K. K.; Ma, L.; Ma, W. J. For. Res. 2019, 30, 1105.
doi: 10.1007/s11676-019-00883-3 |
| [21] |
Sun, N.; Zhang, H.; Sun, P. P.; Khan, A.; Guo, J. H.; Zheng, X. Z.; Sun, Y. G.; Fan, K. H.; Yin, W.; Li, H. Q. Phytomedicine 2020, 77, 153289.
doi: 10.1016/j.phymed.2020.153289 |
| [22] |
Zhang, B.; Liu, Z. Y.; Li, Y. Y.; Luo, Y.; Liu, M. L.; Dong, H. Y.; Wang, Y. X.; Liu, Y.; Zhao, P. T.; Jin, F. G.; Li, Z. C. Eur. J. Pharm. Sci. 2011, 44, 573.
doi: 10.1016/j.ejps.2011.09.020 pmid: 22019524 |
| [23] |
Cheng, X. G.; He, H. Q.; Wang, W. X.; Dong, F. Y.; Zhang, H. H.; Ye, J. M.; Tan, C. C.; Wu, Y. H.; Lv, X. J.; Jiang, X. H.; Qin, X. J. Pest Manage. Sci. 2020, 76, 2711.
doi: 10.1002/ps.v76.8 |
| [24] |
Zhou, S. K.; Zhang, R. L.; Xu, Y. F.; Bi, T. N. Molecules 2012, 17, 6481.
doi: 10.3390/molecules17066481 |
| [25] |
Chen, M. H.; Gu, Y. Y.; Zhang, A. L.; Sze Daniel, M. Y.; Mo, S. L.; May Brian, H. Pharmacol. Res. 2021,171.
|
| [26] |
Zhang, X.; Hou, G. Q.; Liu, A. D.; Xu, H.; Guan, Y.; Wu, Y. S.; Deng, J.; Cao, X. Cell Death Dis. 2019, 10, 10.
doi: 10.1038/s41419-018-1254-x |
| [27] |
Liu, Z.-M.; Yang, X.-L.; Jiang, F.; Pan, Y.-C.; Zhang, L. J. Cell. Biochem. 2019, 121, 3.
|
| [28] |
Hu, J.; Wang, Y. Chin. Arch. Tradit. Chin. Med. 2021, 171. (in Chinese)
|
|
(胡锦丹, 王宇, 中华中医药学刊, 2021, 171.)
|
|
| [29] |
Dai, M.; Cai, Z.; Chen, N.; Li, J.; Wen, J.; Tan, L.; Guo, D. J. South. Med. Univ. 2019, 39, 1239. (in Chinese)
|
|
(戴美琴, 蔡茁, 陈娜娜, 李金州, 温嘉泳, 谭丽转, 郭丹, 南方医科大学学报, 2019, 39, 1239.)
|
|
| [30] |
Yang, J.; He, D.; Peng, Y.; Zhong, H.; Deng, Y.; Yu, Z.; Guan, C.; Zuo, Y.; Xu, Z. OncoTargets Ther. 2017, 10, 5209.
doi: 10.2147/OTT.S149609 pmid: 29138573 |
| [31] |
Fu, S.; Zhao, N.; Jing, G.; Yang, X.; Liu, J.; Zhen, D.; Tang, X. Biomed. Pharmacother. 2020, 128, 110327.
doi: 10.1016/j.biopha.2020.110327 |
| [32] |
Li, Z.; Luo, M. Y.; Cai, B.; Wu, L. C.; Huang, M. T.; Rashid, H.; Jiang, J.; Wang, L. S. Bioorg. Med. Chem. Lett. 2018, 28, 677.
doi: 10.1016/j.bmcl.2018.01.017 |
| [33] |
Wei, J.; Liang, Y.; Wu, L. Molecules 2021, 26, 417.
doi: 10.3390/molecules26020417 |
| [34] |
Sun, X.; Zhuo, X.-B.; Hu, Y.-P.; Zheng, X.; Zhao, Q.-J. Mol. Cell. Biochem. 2018, 449, 47.
doi: 10.1007/s11010-018-3341-9 |
| [35] |
Rashid, H.; Xu, Y.; Muhammad, Y.; Wang, L.; Jiang, J. Eur. J. Med. Chem. 2019, 161, 205.
doi: 10.1016/j.ejmech.2018.10.037 |
| [36] |
Wang, M.; Huang, L.; Su, Y.; Xu, Y.-H.; Huang, L.-Y.; Zhou, X.-Q.; Li, F.-Y. Chemistry 2019, 82, 57. (in Chinese)
|
|
(王妙, 黄琳, 苏燕, 许英红, 黄铃月, 周小群, 李芳耀, 化学通报, 2019, 82, 57.)
|
|
| [37] |
Xin, M.; Pang, F.-H.; Huang, L.; Zhou, X.-Q.; Wang, M.-D.; Li, J.-L.; Li, F.-Y. Chin. J. Synth. Chem. 2020, 28, 483. (in Chinese)
|
|
(辛懋, 庞富华, 黄琳, 周小群, 王萌迪, 李金林, 李芳耀, 合成化学, 2020, 28, 483.)
|
|
| [38] |
Li, F.-Y.; Huang, L.; Li, Q.; Wang, X.; Ma, X. L.; Jiang, C. N.; Zhou, X. Q.; Duan, W. G.; Lei, F. H. Molecules 2019, 24, 4191.
doi: 10.3390/molecules24224191 |
| [39] |
Huang, L.; Huang, R.; Pang, F. H.; Li, A. K.; Huang, G. B.; Zhou, X. Q.; Li, Q.; Li, F. Y.; Ma, X. L. RSC Adv. 2020, 10, 18008.
doi: 10.1039/d0ra02432e pmid: 35517208 |
| [40] |
Li, F. Y.; Huang, L.; Zhou, X. Q.; Li, Q.; Ma, X. L; Duan, W. G.; Wang, X. Chin. J. Org. Chem. 2020, 40, 2845. (in Chinese)
|
|
(李芳耀, 黄琳, 周小群, 李倩, 马献力, 段文贵, 王秀, 有机化学, 2020, 40, 2845.)
doi: 10.6023/cjoc202003062 |
|
| [41] |
Zheng, W. L.; Ma, X. L.; Zhou, X. Q.; Li, F. Y.; Xin, M.; Jiang, C. N. Chem. Ind. For. Prod. 2019, 39, 41. (in Chinese)
|
|
(郑万里, 马献力, 周小群, 李芳耀, 辛懋, 蒋彩娜, 林产化学与工业, 2019, 39, 41.)
|
|
| [42] |
Wang, K.; Zheng, W. L; Jiang, C. N.; Zhou, X. Q.; Li, F. Y.; Xin, M.; Ma, X. L. Chem. Res. Appl. 2020, 32, 1377. (in Chinese)
|
|
(王珂, 郑万里, 蒋彩娜, 周小群, 李芳耀, 辛懋, 马献力, 化学研究与应用, 2020, 32, 1377.)
|
|
| [43] |
Huang, J. L.; Lv, M.; Xu, H. RSC Adv. 2017, 7, 15997.
doi: 10.1039/C7RA00954B |
| [44] |
Xu, H.; Su, X.; Liu, X. Q.; Zhang, K. P.; Hou, Z.; Guo, C. Bioorg. Med. Chem. Lett. 2019, 29, 126726.
doi: 10.1016/j.bmcl.2019.126726 |
| [45] |
Dai, B.; Ma, X.; Tang, Y.; Xu, L.; Guo, S.; Chen, X.; Lu, S.; Wang, G.; Liu, Y. Bioorg. Med. Chem. 2021, 29, 115891.
doi: 10.1016/j.bmc.2020.115891 |
| [46] |
Ly, J. D.; Grubb, D. R.; Lawen, A. Apoptosis 2003, 8, 115.
doi: 10.1023/a:1022945107762 pmid: 12766472 |
| [1] | 邹发凯, 王能中, 姚辉, 王慧, 刘明国, 黄年玉. 1β-/3R-芳基硫代糖的区域与立体选择性合成[J]. 有机化学, 2024, 44(2): 593-604. |
| [2] | 李路瑶, 贺忠文, 张振国, 贾振华, 罗德平. 三芳基碳正离子在有机合成中的应用[J]. 有机化学, 2024, 44(2): 421-437. |
| [3] | 梅青刚, 李清寒. 可见光促进C(3)(杂)芳硫基吲哚化合物的合成研究进展[J]. 有机化学, 2024, 44(2): 398-408. |
| [4] | 冯康博, 陈炯, 古双喜, 王海峰, 陈芬儿. 全连续流反应技术在药物合成中的新进展(2019~2022)[J]. 有机化学, 2024, 44(2): 378-397. |
| [5] | 李鹏辉, 谢青洋, 万福贤, 张元红, 姜林. 含环丙基的新型取代嘧啶-5-甲酰胺的合成及杀菌活性研究[J]. 有机化学, 2024, 44(2): 650-656. |
| [6] | 杨维清, 葛宴兵, 陈元元, 刘萍, 付海燕, 马梦林. 1,8-萘酰亚胺衍生物的设计、合成及其对半胱氨酸的识别研究[J]. 有机化学, 2024, 44(1): 180-194. |
| [7] | 于士航, 刘嘉威, 安碧玉, 边庆花, 王敏, 钟江春. 黑腹尼虎天牛接触性信息素的不对称合成[J]. 有机化学, 2024, 44(1): 301-308. |
| [8] | 赵茜帆, 陈永正, 张世明. 碳基非金属催化剂在有机合成领域的应用及机理研究[J]. 有机化学, 2024, 44(1): 137-147. |
| [9] | 陈珊, 陈志林, 胡琼, 蒙艳双, 黄悦, 陶萍芳, 卢丽如, 黄国保. 含双硫脲基团分子钳在非极性溶剂中识别中性分子[J]. 有机化学, 2024, 44(1): 277-281. |
| [10] | 王化坤, 任晓龙, 宣宜宁. 卤盐催化的α,β-环氧羧酸酯与异氰酸酯[3+2]环加成反应研究[J]. 有机化学, 2024, 44(1): 251-258. |
| [11] | 金玉坤, 任保轶, 梁福顺. 可见光介导的三氟甲基的选择性C-F键断裂及其在偕二氟类化合物合成中的应用[J]. 有机化学, 2024, 44(1): 85-110. |
| [12] | 马翠云, 罗海澜, 张福华, 郭丹, 陈树兴, 王飞. 3-Pyrrolyl BODIPY的绿色生物合成、光物理性质及应用研究[J]. 有机化学, 2024, 44(1): 216-223. |
| [13] | 王博珍, 张婕, 粘春惠, 金茗茗, 孔苗苗, 李物兰, 何文斐, 吴建章. 含有3,4-二氯苯基的酰胺类化合物的合成及抗肿瘤活性研究[J]. 有机化学, 2024, 44(1): 232-241. |
| [14] | 曹瑞霞, 贾玉萍. 含香豆素的吡咯并[2,3-d]嘧啶衍生物的合成及生物活性研究[J]. 有机化学, 2023, 43(9): 3304-3311. |
| [15] | 李焕清, 陈兆华, 陈祖佳, 邱琪雯, 张又才, 陈思鸿, 汪朝阳. 基于有机小分子的汞离子荧光探针研究进展[J]. 有机化学, 2023, 43(9): 3067-3077. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||