有机化学 ›› 2021, Vol. 41 ›› Issue (12): 4815-4824.DOI: 10.6023/cjoc202106014 上一篇 下一篇
所属专题: 有机光催化虚拟合辑; 绿色合成化学专辑; 热点论文虚拟合集
研究论文
耿芳洲a,b, 王世超a, 宋克贤a, 郝文娟a,b,*( ), 姜波a,b,*(
), 姜波a,b,*( )
)
收稿日期:2021-06-07
修回日期:2021-07-14
发布日期:2021-07-19
通讯作者:
郝文娟, 姜波
作者简介:基金资助:
Fangzhou Genga,b, Shichao Wanga, Kexian Songa, Wenjuan Haoa,b( ), Bo Jianga,b(
), Bo Jianga,b( )
)
Received:2021-06-07
Revised:2021-07-14
Published:2021-07-19
Contact:
Wenjuan Hao, Bo Jiang
About author:Supported by:文章分享
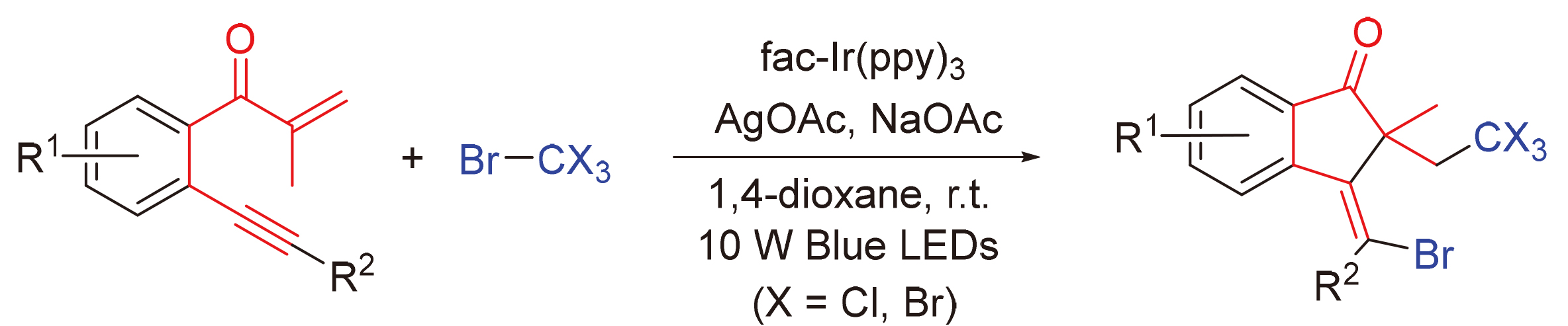
报道了一类新颖的可见光催化的1,6-烯炔参与的Kharasch加成反应. 该反应利用三氯溴甲烷和四溴化碳在光催化还原下能产生三卤代甲基自由基的特性, 实现其在fac-Ir(ppy)3与可见光作用下与1,6-烯炔发生Kharasch加成反应, 区域选择性地合成了具有环外双键以及季碳的1-茚酮衍生物, 产率中等至良好. 基于实验结果及文献报道, 提出了合理的反应机理, 涉及三卤代甲基自由基的原位形成、自由基引发的加成环化以及自由基交叉偶联等. 此外, 该反应具有底物普适性广、高官能团兼容性、100%原子利用率、条件温和、操作简便等优点, 从而为具有潜在应用价值的1-茚酮骨架的构建提供了一种绿色、温和且高效的合成策略, 符合绿色化学理念.
耿芳洲, 王世超, 宋克贤, 郝文娟, 姜波. 可见光催化的1,6-烯炔参与的Kharasch加成反应[J]. 有机化学, 2021, 41(12): 4815-4824.
Fangzhou Geng, Shichao Wang, Kexian Song, Wenjuan Hao, Bo Jiang. Visible-Light-Driven Photocatalytic Kharasch-Type Addition of 1,6-Enynes[J]. Chinese Journal of Organic Chemistry, 2021, 41(12): 4815-4824.
| Entry | Variation of the established conditions | Yield/% |
|---|---|---|
| 1 | None | 85 |
| 2 | 4.0 Equiv. of BrCCl3 | 80 |
| 3 | 3.0 Equiv. of BrCCl3 | 41 |
| 4 | AgSCF3 instead of AgOAc | 73 |
| 5 | 10 mol% of AgOAc | Trace |
| 6 | 50 mol% of AgOAc | 36 |
| 7 | Ru(bpy)3Cl2•6H2O instead of fac-Ir(ppy)3 | 80 |
| 8 | Eosin Y instead of fac-Ir(ppy)3 | 67 |
| 9 | Without fac-Ir(ppy)3 | 0 |
| 10 | No light | 0 |
| 11 | CH3CN as solvent | 72 |
| 12 | THF as solvent | Trace |
| 13 | K2CO3 instead of NaOAc | 64 |
| 14 | CsCO3 instead of NaOAc | 68 |
| Entry | Variation of the established conditions | Yield/% |
|---|---|---|
| 1 | None | 85 |
| 2 | 4.0 Equiv. of BrCCl3 | 80 |
| 3 | 3.0 Equiv. of BrCCl3 | 41 |
| 4 | AgSCF3 instead of AgOAc | 73 |
| 5 | 10 mol% of AgOAc | Trace |
| 6 | 50 mol% of AgOAc | 36 |
| 7 | Ru(bpy)3Cl2•6H2O instead of fac-Ir(ppy)3 | 80 |
| 8 | Eosin Y instead of fac-Ir(ppy)3 | 67 |
| 9 | Without fac-Ir(ppy)3 | 0 |
| 10 | No light | 0 |
| 11 | CH3CN as solvent | 72 |
| 12 | THF as solvent | Trace |
| 13 | K2CO3 instead of NaOAc | 64 |
| 14 | CsCO3 instead of NaOAc | 68 |
| [1] |
(a) Nagle, D. G.; Zhou, Y. D.; Park, P. U.; Paul, V. J.; Rajbhandari, I.; Duncan, C. J. G.; Pasco, D. S. J. Nat. Prod. 2000, 63, 1431.
pmid: 7490731 |
|
(b) Ito, T.; anaka, T.; Iinuma, T. M.; Nakaya, K.; Takahashi, Y.; Sawa, R.; Murata, J.; Darnaedi, D. J. Nat. Prod. 2004, 67, 932.
doi: 10.1021/np030236r pmid: 7490731 |
|
|
(c) Dai, J. R.; Hallock, Y. F.; Cardellina, J. H.; Boyd, M. R. J. Nat. Prod. 1998, 61, 351.
pmid: 7490731 |
|
|
(d) DeSolms, S. J.; Woltersdorf, O. W.; Cragoe, E. J.; Watson, L. S.; Fanelli, G. M. J. Med. Chem. 1978, 21, 437.
doi: 10.1021/jm00203a006 pmid: 7490731 |
|
|
(e) Sugimoto, H.; Iimura, Y.; Yamanishi, Y.; Yamatsu, K. J. Med. Chem. 1995, 38, 4821.
pmid: 7490731 |
|
| [2] |
(a) Anstead, G. M.; Wilson, S. R.; Katzenellenbogen, J. A. J. Med. Chem. 1989, 32, 2163.
pmid: 15876535 |
|
(b) McDevitt, R. E.; Malamas, M. S.; Manas, E. S.; Unwalla, R. J.; Xu, Z. B.; Miller, C. P.; Harris, H. A. Bioorg. Med. Chem. Lett. 2005, 15, 3137.
pmid: 15876535 |
|
|
(c) Park, C. H.; Siomboing, X.; Yous, S.; Gressier, B; Luyckx, M.; Chavatte, P. Eur. J. Med. Chem. 2002, 37, 461-468.
doi: 10.1016/S0223-5234(02)01373-9 pmid: 15876535 |
|
| [3] |
(a) Ahn, J. H.; Shin, M. S.; Jung, S. H.; Kang, S. K.; Kim, K. R.; Dal Rhee, S.; Jung, W. H.; Yang, S. D.; Kim, S. J.; Woo, J. R. J. Med. Chem. 2006, 49, 4781.
doi: 10.1021/jm060389m pmid: 22536944 |
|
(b) Kiselev, E.; DeGuire, S.; Morrell, A.; Agama, K.; Dexheimer, T. S.; Pommier, Y.; Cushman, M. J. Med. Chem. 2011, 54, 6106.
doi: 10.1021/jm200719v pmid: 22536944 |
|
|
(c) Nguyen, T. X.; Morrell, A.; Conda-Sheridan, M.; Marchand, C.; Agama, K.; Bermingam, A.; Stephen, A. G; Chergui, A.; Naumova, A.; Fisher, R.; O’Keefe, B. R.; Pommier, Y.; Cushman, M. J. Med. Chem. 2012, 55, 4457.
doi: 10.1021/jm300335n pmid: 22536944 |
|
|
(d) Gamo, F. J.; Sanz, L. M.; Vidal, J.; de, C. C.; Alvarez, E.; Lavandera, J. L.; Vanderwall, D. E. Green, D. V. S.; Kumar, V.; Hasan, S.; Brown, J. R.; Peishoff, C. E.; Cardon, L. R.; Garcia-Bustos, J. F. Nature 2010, 465, 305.
doi: 10.1038/nature09107 pmid: 22536944 |
|
| [4] |
(a) Liu, W.; Buck, M.; Chen, N.; Shang, M.; Taylor, N. J.; Asoud, J.; Wu, X.; Hasinoff, B. B.; Dmitrienko, G. I. Org. Lett. 2007, 9, 2915.
doi: 10.1021/ol0712374 pmid: 19902905 |
|
(b) Jeffrey, J. L.; Sarpong, R.; Org. Lett. 2009, 11, 5450.
doi: 10.1021/ol902141z pmid: 19902905 |
|
| [5] |
(a) Cappelli, A.; Pericot Mohr, G. L.; Giuliani, G.; Galeazzi, S.; Anzini, M.; Mennuni, L.; Ferrari, F.; Makovec, F.; Kleinrath, E. M.; Langer, T.; Valoti, M.; Giorgi, G.; Vomero, S. J. Med. Chem. 2006, 49, 6451.
pmid: 17064065 |
|
(b) Tseng, C. H.; Tzeng, C. C.; Yang, C. L.; Lu, P. J.; Chen, H. L.; Li, H. Y.; Chuang, Y. C.; Yang, C. N.; Chen, Y. L. J. Med. Chem. 2010, 53, 6169.
pmid: 17064065 |
|
| [6] |
(a) Koelsch, C. F. J. Am. Chem. Soc. 1932, 54, 2487.
doi: 10.1021/ja01345a046 |
|
(b) Frank, R. L.; Eklund, H. J.; Richter, W.; Vanneman, C. R.; Wennerberg, A. N. J. Am. Chem. Soc. 1944, 66, 1.
doi: 10.1021/ja01229a001 |
|
| [7] |
(a) Bergmann, E. D. J. Org. Chem. 1956, 21, 461.
doi: 10.1021/jo01110a023 |
|
(b) Manning, C.; McClory, M. R.; McCullough, J. J. J. Org. Chem. 1981, 46, 919.
doi: 10.1021/jo00318a018 |
|
|
(c) Dong, D.-Q.; Chen, W.-J.; Chen, D.-M.; Li, L. X..; Li, G. H.; Wang, Z. L.; Deng, Q.; Long, S. Chin. J. Org. Chem. 2019, 39, 3190. (in Chinese)
doi: 10.6023/cjoc201904070 |
|
|
( 董道青, 陈文静, 陈德茂, 李丽霞, 李光辉, 王祖利, 邓企, 龙姝, 有机化学, 2019, 39, 3190.)
doi: 10.6023/cjoc201904070 |
|
| [8] |
(a) Chernyak, N.; Gorelsky, S. I.; Gevorgyan, V. Angew. Chem. Int. Ed. 2011, 50, 2342.
doi: 10.1002/anie.201006751 |
|
(b) Shintani, R.; Takatsu, K.; Hayashi, T. Angew. Chem. Int. Ed. 2007, 46, 3735.
doi: 10.1002/(ISSN)1521-3773 |
|
|
(c) He, G.; Wu, C.; Zhou, J.; Yang, Q.; Zhang, C.; Zhou, Y.; Zhang, H.; Liu, H. J. Org. Chem. 2018, 83, 13356.
doi: 10.1021/acs.joc.8b02149 |
|
|
(d) Song, L.; Tian, G.; Van der Eycken, E. V. Chem. Eur. J. 2019, 25, 7645.
doi: 10.1002/chem.v25.32 |
|
|
(e) Liu, Q.-S.; Lv, Y.-F.; Liu, R.-S.; Zhao, X.-H.; Wang, J.-W.; Wei, W. Chin. Chem. Lett. 2021, 32, 136.
doi: 10.1016/j.cclet.2020.11.059 |
|
| [9] |
(a) Zhang, Y. L.; Sun, K.; Lv, Q. Y.; Chen, X. L.; Qu, L. B.; Yu, B. Chin. Chem. Lett. 2019, 30, 1361.
doi: 10.1016/j.cclet.2019.03.034 |
|
(b) Zhang, T.-S.; Hao, W.-J.; Wang, R.; Wang, S.-C.; Tu, S.-J.; Jiang, B. Green Chem. 2020, 22, 4259.
doi: 10.1039/D0GC00771D |
|
|
(c) Shen, Z.-J.; Wu, Y.-N.; He, C.-L.; He, L.; Hao, W.-J.; Wang, A.-F.; Tu, S.-J.; Jiang, B. Chem. Commun. 2018, 54, 445.
doi: 10.1039/C7CC08516H |
|
|
(d) Wu, Y.-N.; Zhang, T.-S.; Hao, W.-J.; Tu, S.-J.; Jiang, B. Asian J. Org. Chem. 2020, 9, 1040.
doi: 10.1002/ajoc.v9.7 |
|
|
(e) Wei, W.-T.; Li, Q.; Zhang, M.-Z.; He, W.-M. Chin. J. Catal. 2021, 42, 731.
doi: 10.1016/S1872-2067(20)63702-0 |
|
|
(f) Zhang, T.-S.; Hao, W.-J.; Cai, P.-J.; Li, G.; Tu, S.-J.; Jiang, B. Front. Chem. 2020, 8, 234.
doi: 10.3389/fchem.2020.00234 |
|
| [10] |
Shi, H.-N.; Huang, M.-H.; Hao, W.-J.; Tu, X.-C.; Tu, S.-J.; Jiang, B. J. Org. Chem. 2019, 84, 16027.
doi: 10.1021/acs.joc.9b02525 pmid: 31769289 |
| [11] |
Shen, Z.-J.; Wang, S.-C.; Hao, W.-J.; Yang, S.-Z.; Tu, S.-J.; Jiang, B. Adv. Synth. Catal. 2019, 361, 3837.
doi: 10.1002/adsc.v361.16 |
| [12] |
(a) Prier, C. K.; Rankic, D. A.; MacMillan, D. W. C. Chem. Rev. 2013, 113, 5322.
doi: 10.1021/cr300503r |
|
(b) Zhu, C.-F.; Zhang, J.; Zhu, Y.-L.; Hao, W.-J.; Tu, S.-J.; Wang, D.-C.; Jiang, B. Org. Chem. Front. 2021, 8, 1952.
doi: 10.1039/D1QO00124H |
|
|
(c) Shi, J.; Wei, W. Chin. J. Org. Chem. 2020, 40, 2170. (in Chinese)
doi: 10.6023/cjoc202000041 |
|
|
( 时建伟, 魏伟, 有机化学, 2020, 40, 2170.)
doi: 10.6023/cjoc202000041 |
|
|
(d) Peng, S.; Lin, Y.-W.; He, W.-M. Chin. J. Org. Chem. 2020, 40, 541. (in Chinese)
doi: 10.6023/cjoc202000006 |
|
|
( 彭莎, 林英武, 何卫民, 有机化学, 2020, 40, 541.)
doi: 10.6023/cjoc202000006 |
|
|
(e) Yi, R.; He, W. Chin. J. Org. Chem. 2021, 41, 1267. (in Chinese)
doi: 10.6023/cjoc202100022 |
|
|
( 易荣楠, 何卫民, 有机化学, 2021, 41, 1267.)
doi: 10.6023/cjoc202100022 |
|
|
(f) Chen, J.-R.; Hu, X. Q.; Lu, L.-Q.; Xiao, W.-J. Chem. Soc. Rev. 2016, 45, 2044.
doi: 10.1039/C5CS00655D |
|
|
(g) Xuan, J.; Zhang, Z. G.; Xiao, W.-J. Angew. Chem. Int. Ed. 2015, 54, 15632.
doi: 10.1002/anie.v54.52 |
|
|
(h) Huang, H. C.; Jia, K. F.; Chen, Y. ACS Catal. 2016, 6, 4983.
doi: 10.1021/acscatal.6b01379 |
|
|
(i) Mi, X.; Kong, Y. F.; Zhang, J. Y.; Pi, C.; Cui, X. L. Chin. Chem. Lett. 2019, 30, 2295.
doi: 10.1016/j.cclet.2019.09.040 |
|
| [13] |
(a) Zhu, S.-S.; Zhou, J.-N.; Wu, Q.-L.; Hao, W.-J.; Tu, S.-J.; Jiang, B. Org. Chem. Front. 2020, 7, 2975.
doi: 10.1039/D0QO00917B pmid: 31769294 |
|
(b) Zhao, Q.; Hao, W.-J.; Shi, H.-N.; Xu, T.; Tu, S.-J.; Jiang, B. Org. Lett. 2019, 21, 9784.
doi: 10.1021/acs.orglett.9b04018 pmid: 31769294 |
|
|
(c) Gui, Q.-W.; Teng, F.; Li, Z.-C.; Xiong, Z.-Y.; Jin, X.-F.; Lin, Y.-W.; Cao, Z.; He, W.-M. Chin. Chem. Lett. 2021, 32, 1907.
doi: 10.1016/j.cclet.2021.01.021 pmid: 31769294 |
|
|
(d) Liu, K.-J.; Wang, Z.; Lu, L.-H.; Chen, J.-Y.; Zeng, F.; Lin, Y.-W.; Cao, Z.; Yu, X.; He, W.-M. Green Chem. 2021, 23, 496.
doi: 10.1039/D0GC02663H pmid: 31769294 |
|
| [14] |
(a) Yu, X.-Y.; Chen, J.-R.; Xiao, W.-J. Chem. Rev. 2021, 121, 506.
doi: 10.1021/acs.chemrev.0c00030 |
|
(b) Yang, Z.; Stivanin, M. L.; Jurberg, I. D.; Koenigs, R. M. Chem. Soc. Rev. 2020, 49, 6833.
doi: 10.1039/D0CS00224K |
|
|
(c) Chen, D. M.; Sun, Y. Y.; Dong, D. Q.; Han, Q. Q.; Wang, Z. L. Chin. J. Org. Chem. 2020, 40, 4267. (in Chinese)
doi: 10.6023/cjoc202006025 |
|
|
( 陈德茂, 孙媛媛, 董道青, 韩晴晴, 王祖利, 有机化学, 2020, 40, 4267.)
doi: 10.6023/cjoc202006025 |
|
|
(d) Meng, N.; Lv, Y. F.; Liu, Q. S.; Liu, R. S.; Zhao, X. H.; Wei, W. Chin. Chem. Lett. 2021, 32, 258.
doi: 10.1016/j.cclet.2020.11.034 |
|
| [15] |
Wu, D.; Hao, W.-J.; Rao, Q.; Lu, Y.; Tu, S.-J.; Jiang, B. Chem. Commun. 2021, 57, 1911.
doi: 10.1039/D0CC07880H |
| [16] |
(a) Kharasch, M. S.; Jensen, E. V.; Urry, W. H. Science 1945, 102, 128.
pmid: 17777366 |
|
(b) Chen, B.; Fang, C.; Liu, P.; Ready, J. M. Angew. Chem., nt. Ed. 2017, 56, 8780.
pmid: 17777366 |
|
|
(c) Wang, L. L.; Zhang, M.; Zhang, Y. L.; Liu, Q. S.; Zhang, X. H.; Li, J. S.; Luo, Z. D.; Wei, W. Chin. Chem. Lett. 2020, 31, 67.
doi: 10.1016/j.cclet.2019.05.041 pmid: 17777366 |
|
| [17] |
(a) Bacauanu, V.; Cardinal, S.; Yamauchi, M.; Kondo, M.; Fernandez, D. F.; Remy, R.; MacMillan, D. W. C. Angew. Chem., nt. Ed. 2018, 57, 12543.
|
|
(b) Wang, S.-W.; Yu, J.; Zhou, Q.-Y.; Chen, S.-Y.; Xu, Z.-H. Tang, S. ACS Sustainable Chem. Eng. 2019, 7, 10154.
doi: 10.1021/acssuschemeng.9b02178 |
| [1] | 赵瑜, 段玉荣, 史时辉, 白育斌, 黄亮珠, 杨晓军, 张琰图, 冯彬, 张建波, 张秋禹. 可见光促进高价碘(III)试剂参与反应的研究进展[J]. 有机化学, 2023, 43(12): 4106-4140. |
| [2] | 季晓霜, 付荣, 王树良, 郝文娟, 姜波. 可见光驱动酚/芳胺联-1,6-烯炔与全卤代甲烷的Kharasch反应[J]. 有机化学, 2022, 42(12): 4282-4291. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||