有机化学 ›› 2022, Vol. 42 ›› Issue (3): 819-829.DOI: 10.6023/cjoc202107064 上一篇 下一篇
研究论文
收稿日期:2021-07-30
修回日期:2021-10-12
发布日期:2021-11-17
通讯作者:
罗稳, 田智勇
基金资助:
Yongmei Zhaoa, Yeshu Mub, Wen Luob( ), Zhiyong Tianc(
), Zhiyong Tianc( )
)
Received:2021-07-30
Revised:2021-10-12
Published:2021-11-17
Contact:
Wen Luo, Zhiyong Tian
Supported by:文章分享
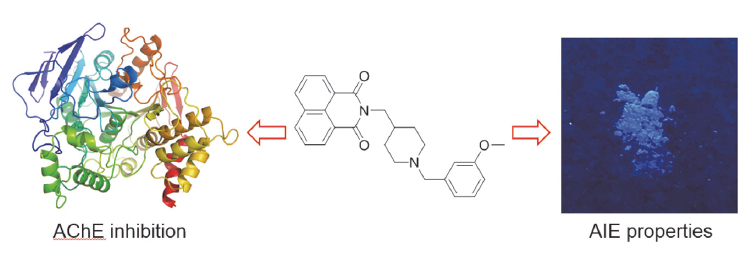
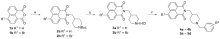
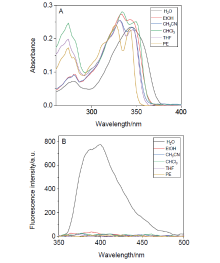
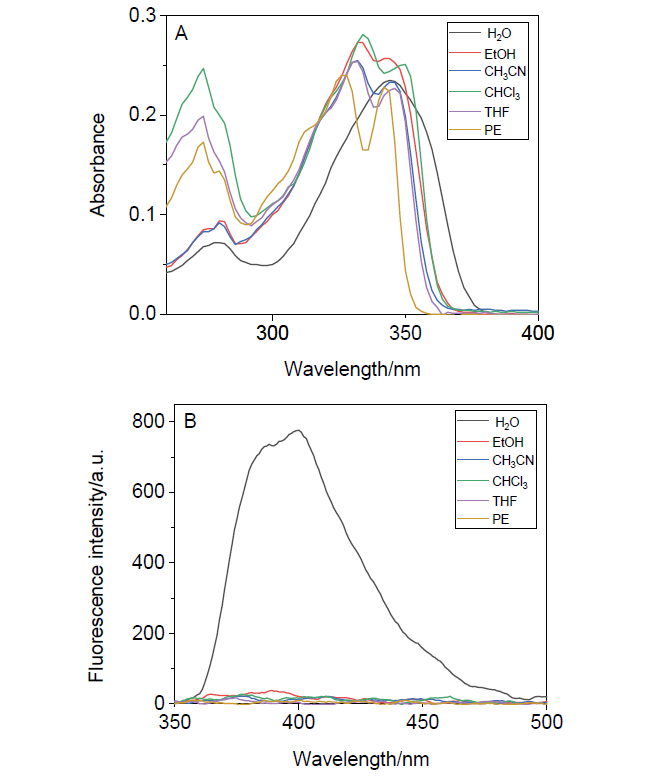
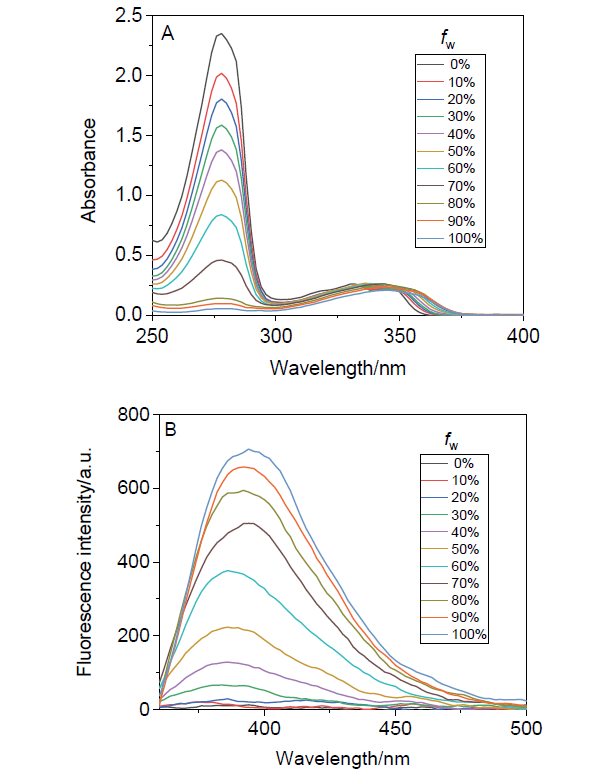
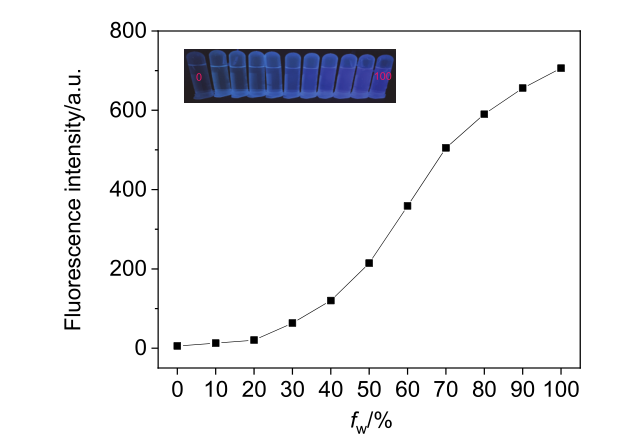
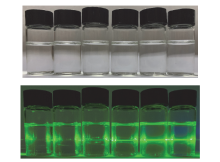
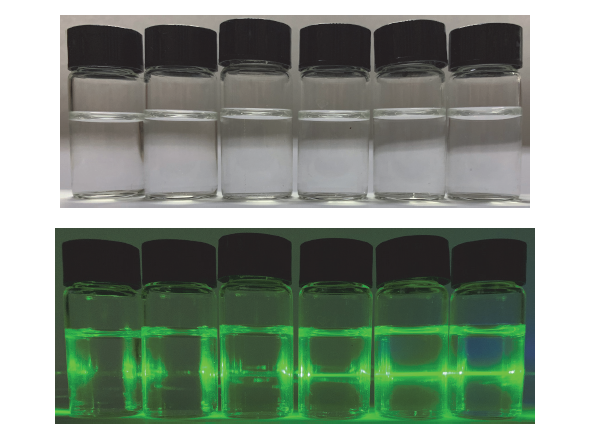
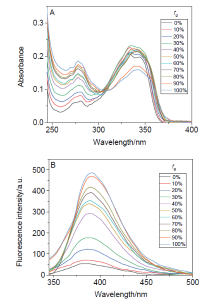
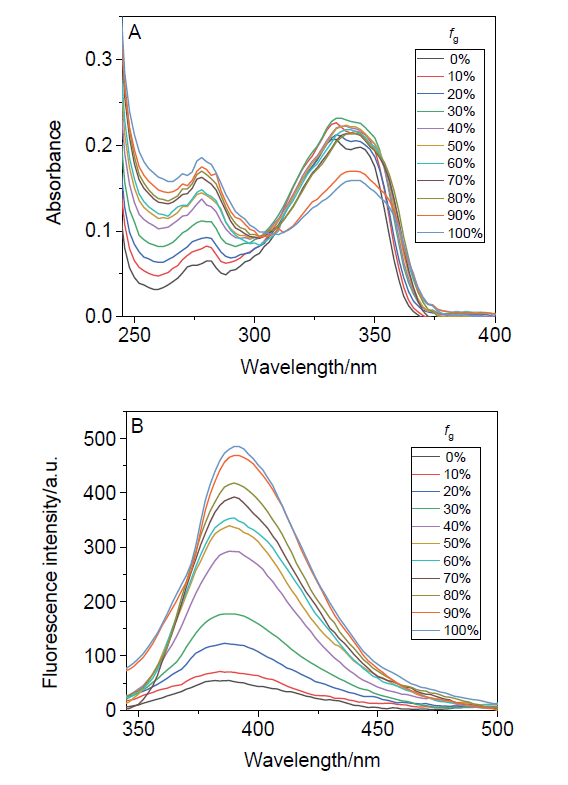
阿尔茨海默症(Alzheimer’s disease, AD)是一种神经退行性疾病, 严重影响老年人的生活质量, 目前治疗AD的药物主要是胆碱酯酶抑制剂, 如多奈哌齐、卡巴拉汀等. 本文基于多奈哌齐结构, 设计合成了一系列新的萘酰亚胺衍生物并进行了活性评价. 结果表明, 所合成的化合物均对乙酰胆碱酯酶(AChE)有选择性抑制, 其中2-((1-(3-甲氧基苄基)哌啶-4-基)甲基)-1H-苯并异喹啉-1,3(2H)-二酮(4k)的抑制活性最强, IC50值为4.43 μmol•L–1, 优于对照药物卡巴拉汀. 酶动力学及分子对接表明4k能够同时作用于AChE的催化活性位点和外周结合位点, 并且4k对SH-SY5Y和PC12细胞毒性较低. 此外, 这些化合物均显示出典型的聚集诱导发光(AIE)性质, 可能与萘酰亚胺分子内旋转受阻机制有关.
赵永梅, 穆叶舒, 罗稳, 田智勇. 胆碱酯酶抑制剂萘酰亚胺衍生物的合成与聚集诱导发光性质[J]. 有机化学, 2022, 42(3): 819-829.
Yongmei Zhao, Yeshu Mu, Wen Luo, Zhiyong Tian. Synthesis of Naphthalimide Derivatives as Cholinesterase Inhibitors with Aggregation Induced Emission Properties[J]. Chinese Journal of Organic Chemistry, 2022, 42(3): 819-829.
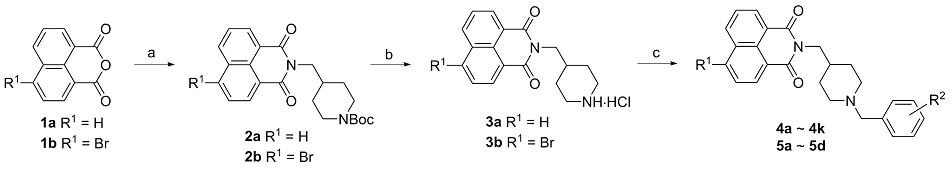
| Compd. | R1 | | IC50/(μmol•L–1) for AChEa | IC50/(μmol•L–1) for BChEb | SIc |
|---|---|---|---|---|---|
| 4a | H | | 18.43±1.96 | >100 | <0.18 |
| 4b | H | | 12.28±1.79 | >100 | <0.12 |
| 4c | H | | 9.15±0.55 | >100 | <0.09 |
| 4d | H | | 11.19±1.13 | >100 | <0.11 |
| 4e | H | | 4.66±0.54 | >100 | <0.05 |
| 4f | H | | 12.98±1.78 | >100 | <0.13 |
| 4g | H | | 8.86±1.05 | >100 | <0.09 |
| 4h | H | | 7.43±0.71 | >100 | <0.07 |
| 4i | H | | 12.72±1.92 | >100 | <0.13 |
| 4j | H | | 11.57±1.06 | >100 | <0.12 |
| 4k | H | | 4.43±0.45 | >100 | <0.04 |
| 5a | Br | | 17.14±1.69 | >100 | <0.17 |
| 5b | Br | | 14.52±0.75 | >100 | <0.15 |
| 5c | Br | | 9.58±0.81 | 41.36±2.23 | 0.23 |
| 5d | Br | | 14.30±1.30 | >100 | <0.14 |
| Riv. d | — | — | 7.25±0.34 | 1.85±0.21 | 3.92 |
| Dpz. e | — | — | 0.027±0.003 | 1.155±0.03 | 0.023 |
| Compd. | R1 | | IC50/(μmol•L–1) for AChEa | IC50/(μmol•L–1) for BChEb | SIc |
|---|---|---|---|---|---|
| 4a | H | | 18.43±1.96 | >100 | <0.18 |
| 4b | H | | 12.28±1.79 | >100 | <0.12 |
| 4c | H | | 9.15±0.55 | >100 | <0.09 |
| 4d | H | | 11.19±1.13 | >100 | <0.11 |
| 4e | H | | 4.66±0.54 | >100 | <0.05 |
| 4f | H | | 12.98±1.78 | >100 | <0.13 |
| 4g | H | | 8.86±1.05 | >100 | <0.09 |
| 4h | H | | 7.43±0.71 | >100 | <0.07 |
| 4i | H | | 12.72±1.92 | >100 | <0.13 |
| 4j | H | | 11.57±1.06 | >100 | <0.12 |
| 4k | H | | 4.43±0.45 | >100 | <0.04 |
| 5a | Br | | 17.14±1.69 | >100 | <0.17 |
| 5b | Br | | 14.52±0.75 | >100 | <0.15 |
| 5c | Br | | 9.58±0.81 | 41.36±2.23 | 0.23 |
| 5d | Br | | 14.30±1.30 | >100 | <0.14 |
| Riv. d | — | — | 7.25±0.34 | 1.85±0.21 | 3.92 |
| Dpz. e | — | — | 0.027±0.003 | 1.155±0.03 | 0.023 |




| [1] |
https://finance.sina.com.cn/china/gncj/2021-05-11/doc-ikmxzfmm1763811.shtml.
|
| [2] |
Scarpini, E.; Scheltens, P.; Feldman, H. Lancet Neurol. 2003, 2, 539.
pmid: 12941576 |
| [3] |
Mimica, N.; Presečki, P. Psychiatr. Danub. 2009, 21, 108.
|
| [4] |
Du, C.-Q.; Xie, B.-H.; He, M.; Hu, Z.-Y.; Liu, Y.; He, X.; Liu, F.-Y.; Cheng, C.; Zhou, H.-B.; Huang, S.-T.; Dong, C.-E. Chin. J. Org. Chem. 2020, 40, 2035. (in Chinese)
doi: 10.6023/cjoc202002039 |
|
(杜川黔, 谢宝花, 贺明, 胡志烨, 刘豫, 何雪, 刘凡玉, 程晨, 周海兵, 黄胜堂, 董春娥, 有机化学, 2020, 40, 2035.)
doi: 10.6023/cjoc202002039 |
|
| [5] |
Zheng, S.-Y.; Yu, C.-H.; Shen, Z.-W. Chin. Chin. J. Org. Chem. 2013, 33, 2261. (in Chinese)
doi: 10.6023/cjoc201306006 |
|
(郑书岩, 郁春辉, 沈征武, 有机化学, 2013, 33, 2261.)
doi: 10.6023/cjoc201306006 |
|
| [6] |
Bortolami, M.; Rocco, D.; Messore, A.; Di Santo, R.; Costi, R.; Madia, V. N.; Scipione, L.; Pandolfi, F. Expert Opin. Ther. Pat. 2021, 31, 399.
doi: 10.1080/13543776.2021.1874344 pmid: 33428491 |
| [7] |
Sang, Z.; Qiang, X.; Li, Y.; Xu, R.; Cao, Z.; Song, Q.; Wang, T.; Zhang, X.; Liu, H.; Tan, Z.; Deng, Y. Eur. J. Med. Chem. 2017, 135, 307.
doi: 10.1016/j.ejmech.2017.04.054 |
| [8] |
Xu, Z.; Xiao, Y.; Qian, X.; Cui, J.; Cui, D. Org. Lett. 2005, 7, 889.
doi: 10.1021/ol0473445 |
| [9] |
Duke, R. M.; Veale, E. B.; Pfeffer, F. M.; Kruger, P. E.; Gunnlaugsson, T. Chem. Soc. Rev. 2010, 39, 3936.
doi: 10.1039/b910560n |
| [10] |
Xie, Z.-D.; Fu, M.-L.; Yin, B.; Zhu, Q. Chin. J. Org. Chem. 2018, 38, 1364. (in Chinese)
doi: 10.6023/cjoc201712031 |
|
(谢振达, 付曼琳, 尹彪, 朱勍, 有机化学, 2018, 38, 1364.)
doi: 10.6023/cjoc201712031 |
|
| [11] |
Tomczyk, M. D.; Walczak, K. Z. Eur. J. Med. Chem. 2018, 159, 393.
doi: 10.1016/j.ejmech.2018.09.055 |
| [12] |
Stone, R. M.; Mazzola, E.; Neuberg, D.; Allen, S. L.; Pigneux, A.; Stuart, R. K.; Wetzler, M.; Rizzieri, D.; Erba, H. P.; Damon, L.; Jang, J. H.; Tallman, M. S.; Warzocha, K.; Masszi, T.; Sekeres, M. A.; Egyed, M.; Horst, H. A.; Selleslag, D.; Solomon, S. R.; Venugopal, P.; Lundberg, A. S.; Powell, B. J. Clin. Oncol. 2015, 33, 1252.
doi: 10.1200/JCO.2014.57.0952 |
| [13] |
Chen, Z.; Xu, Y.; Qian, X. Chin. Chem. Lett. 2018, 29, 1741.
doi: 10.1016/j.cclet.2018.09.020 |
| [14] |
Tandon, R.; Luxami, V.; Kaur, H.; Tandon, N.; Paul, K. Chem. Rec. 2017, 17, 956.
doi: 10.1002/tcr.v17.10 |
| [15] |
Rong, R.-X.; Li, J.-M.; Li, Y.-F.; Guo, X.-Y.; Wang, C.; Li, Y.-J.; Li, J.-M.; Han, B.-J.; Cao, Z.-R.; Wang, K.-R.; Li, X.-L. Chin. J. Org. Chem. 2021, 41, 1599. (in Chinese)
doi: 10.6023/cjoc202008015 |
|
(戎瑞雪, 李基民, 李耀文, 郭啸宇, 王冲, 李艳军, 李金梅, 韩宝君, 曹志然, 王克让, 李小六, 有机化学, 2021, 41, 1599.)
doi: 10.6023/cjoc202008015 |
|
| [16] |
Luo, J.; Xie, Z.; Lam, J. W.; Cheng, L.; Chen, H.; Qiu, C.; Kwok, H. S.; Zhan, X.; Liu, Y.; Zhu, D.; Tang, B. Z. Chem. Commun. 2001, 18, 1740.
|
| [17] |
Gopikrishna, P.; Meher, N.; Iyer, P. K. ACS Appl. Mater. Interfaces 2018, 10, 12081.
doi: 10.1021/acsami.7b14473 |
| [18] |
Ma, Y.; Zhao, L.; Li, Y.; Liu, J.; Yang, Y.; Chu, T. Tetrahedron 2018, 74, 2684.
doi: 10.1016/j.tet.2018.04.034 |
| [19] |
Srivastava, A. K.; Singh, A. K.; Kumari, N.; Yadav, R.; Gulino, A.; Speghini, A.; Nagarajan, R.; Mishra, L. J. Lumin. 2017, 182, 274.
doi: 10.1016/j.jlumin.2016.10.042 |
| [20] |
Ma, X.; Chi, W.; Han, X.; Wang, C.; Liu, S.; Liu, X.; Yin, J. Chin. Chem. Lett. 2021, 32, 1790.
doi: 10.1016/j.cclet.2020.12.031 |
| [21] |
Gao, J.; Midde, N.; Zhu, J.; Terry, A. V.; McInnes, C.; Chapman, J. M. Bioorg. Med. Chem. Lett. 2016, 26, 5573.
doi: 10.1016/j.bmcl.2016.09.072 |
| [22] |
Blaikie, L.; Kay, G.; Kong Thoo Lin, P. Bioorg. Med. Chem. Lett. 2020, 30, 127505.
doi: 10.1016/j.bmcl.2020.127505 |
| [23] |
Gao, J.; Suo, C.; Tseng, J. H.; Moss, M. A.; Terry, A. V., Jr.; Chapman, J. Int. J. Mol. Sci. 2021, 22, 3120.
doi: 10.3390/ijms22063120 |
| [24] |
Dai, F.; Li, Q.; Wang, Y.; Ge, C.; Feng, C.; Xie, S.; He, H.; Xu, X.; Wang, C. J. Med. Chem. 2017, 60, 2071.
doi: 10.1021/acs.jmedchem.6b01846 |
| [25] |
Li, M.; Wang, Y.; Ge, C.; Chang, L.; Wang, C.; Tian, Z.; Wang, S.; Dai, F.; Zhao, L.; Xie, S. Eur. J. Med. Chem. 2018, 143, 1732.
doi: 10.1016/j.ejmech.2017.10.069 |
| [26] |
Alonso, D.; Dorronsoro, I.; Rubio, L.; Muñoz, P.; García-Palomero, E.; Monte, M. D.; Bidon-Chanal, A.; Orozco, M.; Luque, F. J.; Castro, A.; Medina, M.; Martínez, A. Bioorg. Med. Chem. 2005, 13, 6588.
doi: 10.1016/j.bmc.2005.09.029 |
| [27] |
Ellman, G. L.; Courtney, K. D.; Andres Jr, V.; Feather-Stone, R. M. Biochem. Pharmacol. 1961, 7, 88.
doi: 10.1016/0006-2952(61)90145-9 |
| [28] |
Elsinghorst, P. W.; Tanarro, C. M.; Gutschow, M. J. Med. Chem. 2006, 49, 7540.
pmid: 17149883 |
| [29] |
Mao, F.; Chen, J.; Zhou, Q.; Luo, Z.; Huang, L.; Li, X. Bioorg. Med. Chem. Lett. 2013, 23, 6737.
doi: 10.1016/j.bmcl.2013.10.034 |
| [30] |
Sukumaran, S. D.; Nasir, S. B.; Tee, J. T.; Buckle, M. J. C.; Othman, R.; Rahman, N. A.; Lee, V. S.; Bukhari, S. N. A.; Chee, C. F. J. Enzyme Inhib. Med. Chem. 2021, 36, 130.
doi: 10.1080/14756366.2020.1847100 |
| [1] | 杨维清, 葛宴兵, 陈元元, 刘萍, 付海燕, 马梦林. 1,8-萘酰亚胺衍生物的设计、合成及其对半胱氨酸的识别研究[J]. 有机化学, 2024, 44(1): 180-194. |
| [2] | 赵洋, 陈盼盼, 韩立志, 王恩举. 三苯基咪唑衍生物的聚集诱导发光性质及其细胞成像应用[J]. 有机化学, 2023, 43(7): 2454-2461. |
| [3] | 刘铃, 浩涛涛, 伍晚花, 杨成. 利用超分子策略构筑具有聚集诱导发光(AIE)功能的二苯乙烯型分子开关[J]. 有机化学, 2023, 43(6): 2189-2196. |
| [4] | 周五, 彭敏, 梁庆祥, 吴爱斌, 舒文明, 余维初. 高选择和高灵敏检测溶液和气相中硫化氢的新型萘酰亚胺类开启型荧光探针[J]. 有机化学, 2023, 43(12): 4277-4283. |
| [5] | 张越华, 聂飞, 周路, 王晓烽, 刘源, 霍延平, 陈文铖, 赵祖金. 苯并噻唑酮类热活化延迟荧光材料的合成及其光电性能研究[J]. 有机化学, 2023, 43(11): 3876-3887. |
| [6] | 刘梦, 黄延茹, 孙小飞, 汤立军. 一种基于“聚集诱导发光+激发态分子内质子转移”机制的苯并噻唑衍生物荧光探针及其对次氯酸根的识别[J]. 有机化学, 2023, 43(1): 345-351. |
| [7] | 李阳阳, 孙小飞, 胡晓玲, 任源远, 钟克利, 燕小梅, 汤立军. 三苯胺衍生物的合成及其基于聚集诱导发光(AIE)机理对汞离子“OFF-ON”荧光识别[J]. 有机化学, 2023, 43(1): 320-325. |
| [8] | 张继东, 颜婉琳, 胡文强, 郭典, 张大龙, 权校昕, 卜贤盼, 陈思宇. 一种具有聚集诱导发光性能的Zn2+荧光探针的设计合成[J]. 有机化学, 2023, 43(1): 326-331. |
| [9] | 师春甜, 余美, 吴爱斌, 罗江雄, 黎小军, 王宁晨, 舒文明, 余维初. 水溶性萘酰亚胺类荧光探针对Fe3+和$\text{C}{{\text{r}}_{2}}\text{O}_{7}^{2-}$的特异性检测[J]. 有机化学, 2022, 42(9): 2806-2813. |
| [10] | 夏伟康, 刘闯, 叶盛, 汪磊, 刘瑞源. 含磺酰胺基团苯并噻二唑荧光染料的合成及其肿瘤细胞长效示踪应用研究[J]. 有机化学, 2022, 42(8): 2535-2541. |
| [11] | 陈兆华, 曹西颖, 陈思鸿, 宇世伟, 林燕兰, 林舒婷, 汪朝阳. 三取代烯烃型聚集诱导发光分子的设计、合成与应用[J]. 有机化学, 2022, 42(8): 2355-2363. |
| [12] | 郭泽, 吴迪, 王丽丽, 段征. BF3•Et2O促进的双烯酮-酚重排合成具有聚集诱导发光(AIE)效应的磷杂七元环化合物[J]. 有机化学, 2022, 42(8): 2481-2487. |
| [13] | 郭钺甜, 潘永鑫, 汤立军. 聚集诱导发光(AIE)和激发态分子内质子转移(ESIPT)结构融合的反应型荧光探针的研究进展[J]. 有机化学, 2022, 42(6): 1640-1650. |
| [14] | 卢辉旭, 唐永和, 周红梅, 林伟英. 一种荧光增强型甲醛荧光探针的合成及其性能研究[J]. 有机化学, 2022, 42(4): 1163-1169. |
| [15] | 丁伟, 程勃雯, 王萌, 窦清玉, 李思颖, 张鹏, 罗千福. 基于有机光致变色的聚集诱导发光分子的研究进展[J]. 有机化学, 2022, 42(2): 363-383. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||