有机化学 ›› 2024, Vol. 44 ›› Issue (1): 180-194.DOI: 10.6023/cjoc202306016 上一篇 下一篇
研究论文
杨维清a, 葛宴兵a, 陈元元a, 刘萍a, 付海燕b, 马梦林a,b,*( )
)
收稿日期:2023-06-19
修回日期:2023-09-04
发布日期:2023-09-21
基金资助:
Weiqing Yanga, Yanbing Gea, Yuanyuan Chena, Ping Liua, Haiyan Fub, Menglin Maa,b( )
)
Received:2023-06-19
Revised:2023-09-04
Published:2023-09-21
Contact:
*E-mail: Supported by:文章分享

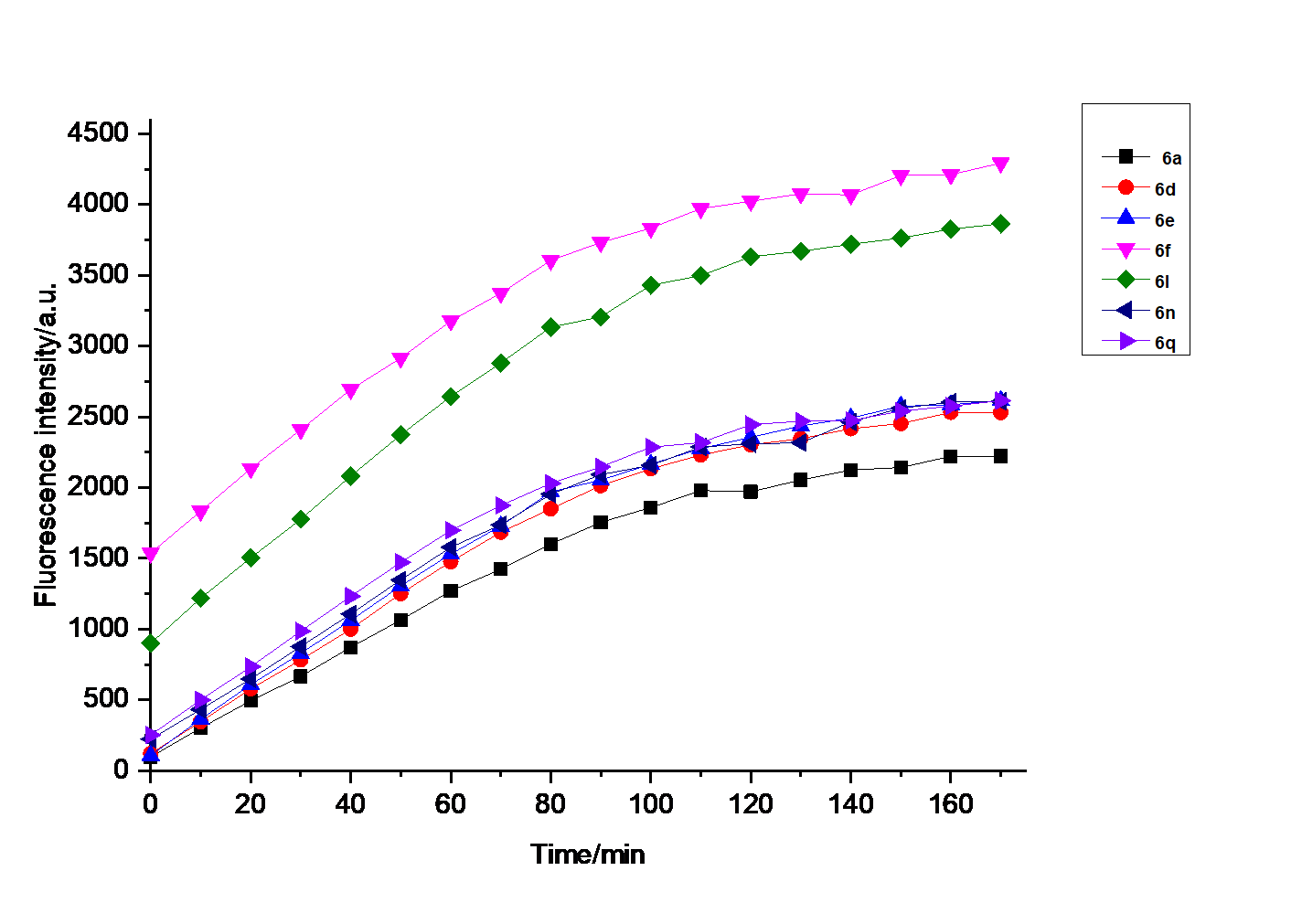
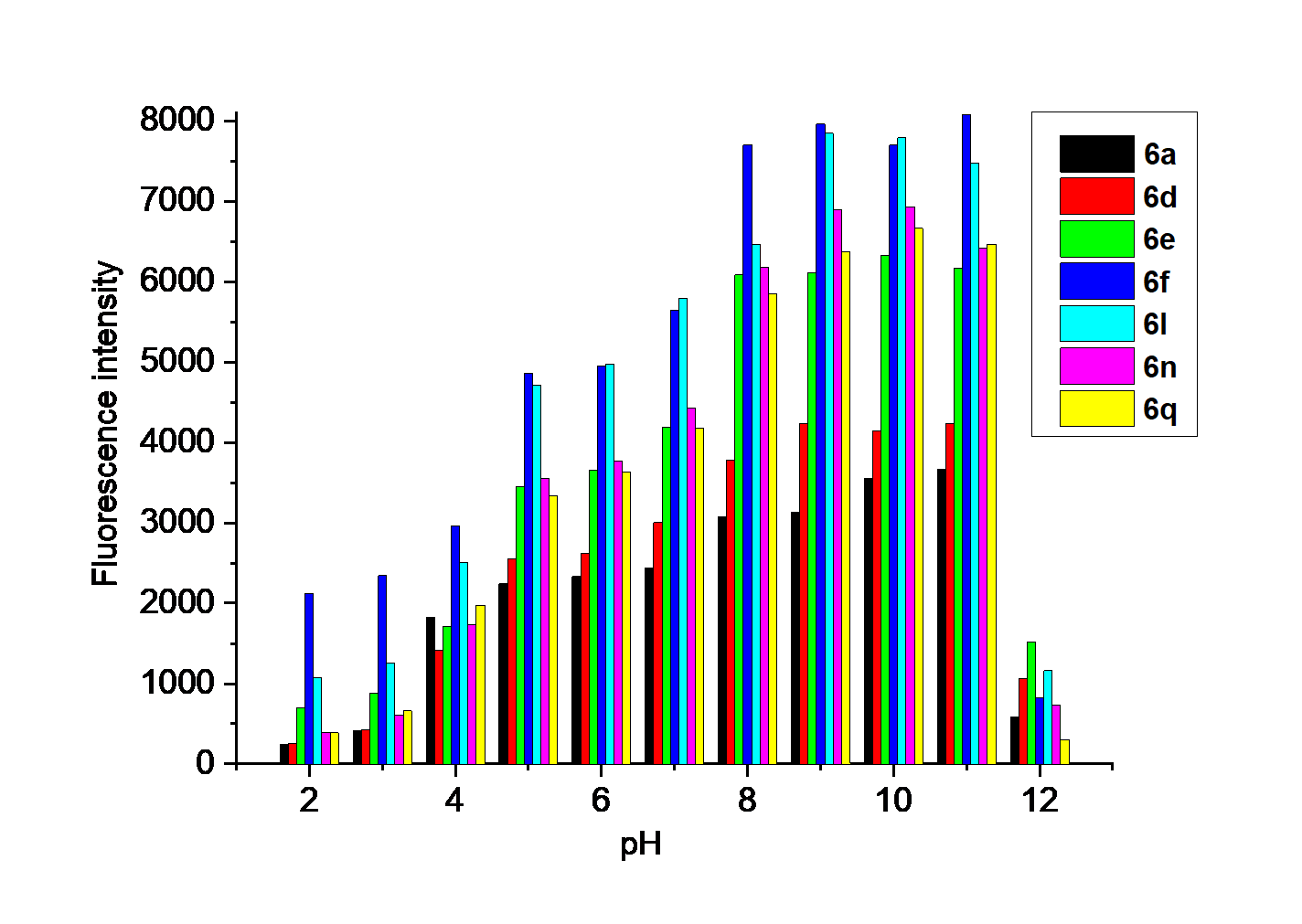
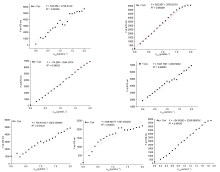
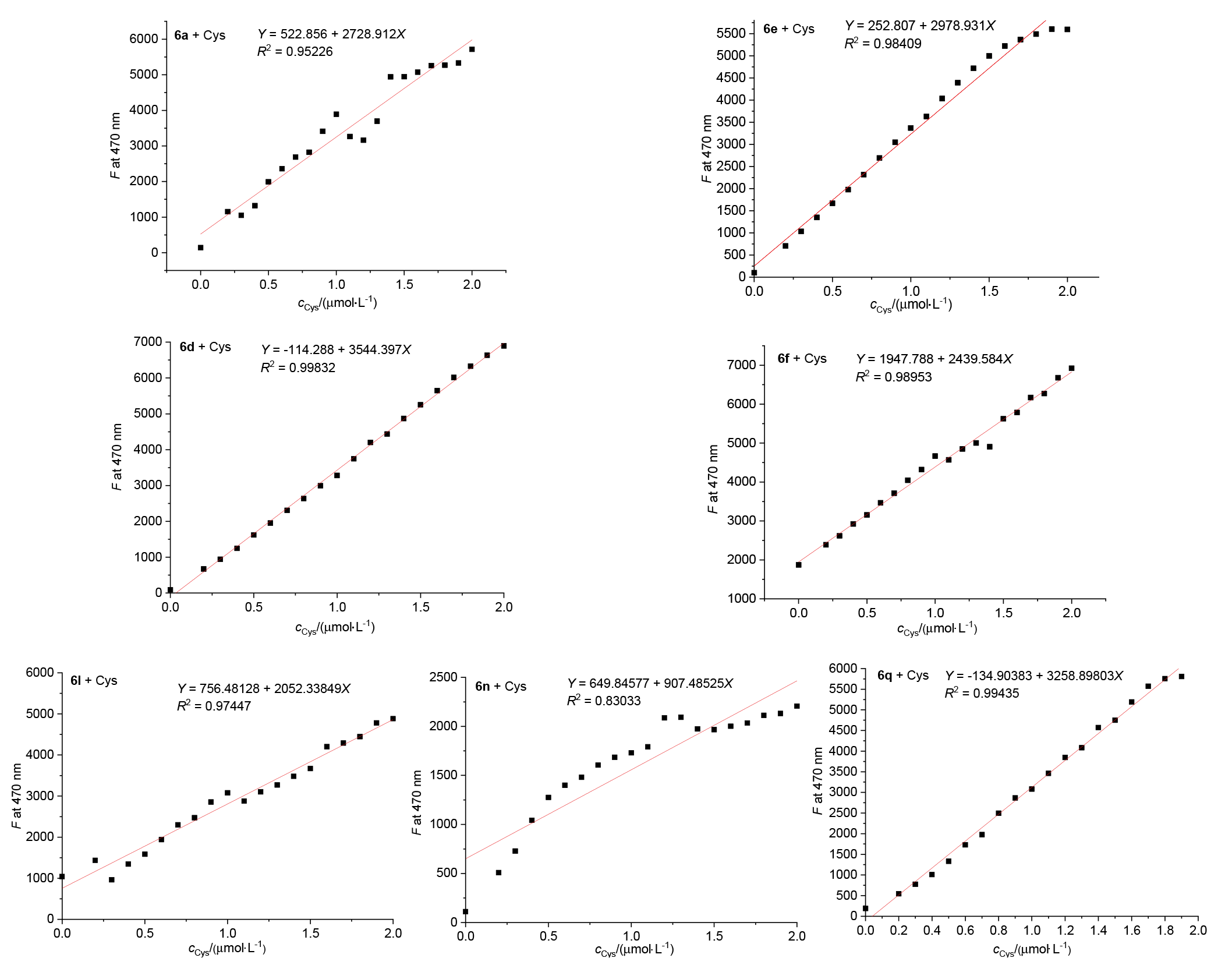
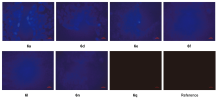
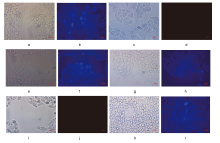
巯基氨基酸水平异常与多种疾病相关, 其检测仍存在一定的局限, 研究检测巯基氨基酸的荧光探针具有一定的价值. 以苊为原料合成了61个1,8-萘酰亚胺衍生物, 研究了该类化合物的荧光性能及其作为半胱氨酸含量测定的荧光探针的可能性. 紫外光谱分析表明1,8-萘酰亚胺衍生物上N-取代基对最大吸收波长无明显影响; 荧光光谱(FL)的性能测试显示硝基萘酰亚胺衍生物N-甲基-4-硝基-1,8-萘二甲酰亚胺(4a)~4-硝基-N-间氟苯基-1,8-萘二甲酰亚胺(4s)无荧光, 氨基萘酰亚胺衍生物N-甲基-4-氨基-1,8-萘二甲酰亚胺(5a)~4-氨基-N-间氟苯基-1,8-萘二甲酰亚胺(5s)有强烈黄色荧光, 而马来酰亚胺萘酰亚胺衍生物N-甲基-4-(1H-吡咯-2,5-二酮-1-基)-1,8-萘二甲酰亚胺(6a)~4-(1H-吡咯-2,5-二酮-1-基)-N-(间氟苯基)-1,8-萘二甲酰亚胺(6s)有微弱蓝色荧光, 其中7个马来酰亚胺萘酰亚胺衍生物探针对半胱氨酸(Cys)溶液有荧光点亮效应. 对7个探针加入21种其它氨基酸作为干扰项的测试显示探针对半胱氨酸检测有良好的选择性. 研究了不同pH值下荧光强度, 检测探针与半胱氨酸的响应时间, 以及探针溶液荧光强度随氨基酸浓度的变化, 其线性相关系数均达到0.95以上, 探针对半胱氨酸的各项指标均表现出了较好的灵敏性. Hela细胞荧光成像验证了7个荧光探针应用于细胞内半胱氨酸的检测同样效果良好.
杨维清, 葛宴兵, 陈元元, 刘萍, 付海燕, 马梦林. 1,8-萘酰亚胺衍生物的设计、合成及其对半胱氨酸的识别研究[J]. 有机化学, 2024, 44(1): 180-194.
Weiqing Yang, Yanbing Ge, Yuanyuan Chen, Ping Liu, Haiyan Fu, Menglin Ma. Design and Synthesis of Fluorescent 1,8-Napthalimide Derivatives and Their Identification of Cysteine[J]. Chinese Journal of Organic Chemistry, 2024, 44(1): 180-194.
| Compd. | λmax/nm | ε/(103 L• mol-¹•cm-¹) | Compd. | λmax/nm | ε/(103 L• mol-¹•cm-¹) | Compd. | λmax/nm | ε/(103 L• mol-¹•cm-¹) | Compd. | λmax/nm | ε/(103 L• mol-¹•cm-¹) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 4a | 353 | 10.78 | 5a | 436 | 13.60 | 6a | 338 | 17.20 | 6a+Cys | 348 | 16.25 |
| 4b | 352 | 10.86 | 5b | 436 | 21.15 | 6b | 346 | 16.10 | 6b+Cys | 366 | 9.60 |
| 4c | 352 | 7.64 | 5c | 434 | 11.40 | 6c | 338 | 17.85 | 6c+Cys | 344 | 15.90 |
| 4d | 350 | 11.29 | 5d | 437 | 13.70 | 6d | 353 | 9.00 | 6d+Cys | 351 | 7.45 |
| 4e | 354 | 14.85 | 5e | 441 | 10.15 | 6e | 354 | 9.75 | 6e+Cys | 355 | 8.55 |
| 4f | 352 | 13.70 | 5f | 436 | 10.55 | 6f | 352 | 7.10 | 6f+Cys | 351 | 5.70 |
| 4g | 354 | 13.50 | 5g | 437 | 8.10 | 6g | 344 | 9.55 | 6g+Cys | 348 | 10.60 |
| 4h | 355 | 12.60 | 5h | 440 | 18.40 | 6h | 346 | 7.30 | 6h+Cys | 344 | 8.20 |
| 4i | 354 | 4.45 | 5i | 434 | 10.85 | 6i | 347 | 10.80 | 6i+Cys | 347 | 8.30 |
| 4j | 354 | 8.90 | 5j | 436 | 12.10 | 6j | 344 | 12.25 | 6j+Cys | 349 | 13.30 |
| 4k | 354 | 11.95 | 5k | 437 | 9.65 | 6k | 344 | 15.85 | 6k+Cys | 348 | 14.60 |
| 4l | 356 | 12.70 | 5l | 438 | 10.75 | 6l | 348 | 13.20 | 6l+Cys | 349 | 12.10 |
| 4m | 354 | 11.45 | 5m | 437 | 8.80 | 6m | 364 | 15.05 | 6m+Cys | 364 | 15.90 |
| 4n | 353 | 13.45 | 5n | 437 | 12.15 | 6n | 348 | 14.25 | 6n+Cys | 354 | 14.20 |
| 4o | 357 | 14.15 | 5o | 438 | 15.15 | 6o | 345 | 13.35 | 6o+Cys | 359 | 13.10 |
| 4p | 357 | 13.80 | 5p | 437 | 19.25 | 6p | 345 | 12.40 | 6p+Cys | 345 | 11.15 |
| 4q | 359 | 11.90 | 5q | 437 | 17.10 | 6q | 345 | 10.45 | 6q+Cys | 365 | 9.70 |
| 4r | 352 | 8.75 | 5r | 437 | 30.25 | 6r | 342 | 12.15 | 6r+Cys | 359 | 10.55 |
| 4s | 353 | 5.10 | 5s | 436 | 7.55 | 6s | 341 | 9.05 | 6s+Cys | 340 | 8.65 |
| Compd. | λmax/nm | ε/(103 L• mol-¹•cm-¹) | Compd. | λmax/nm | ε/(103 L• mol-¹•cm-¹) | Compd. | λmax/nm | ε/(103 L• mol-¹•cm-¹) | Compd. | λmax/nm | ε/(103 L• mol-¹•cm-¹) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 4a | 353 | 10.78 | 5a | 436 | 13.60 | 6a | 338 | 17.20 | 6a+Cys | 348 | 16.25 |
| 4b | 352 | 10.86 | 5b | 436 | 21.15 | 6b | 346 | 16.10 | 6b+Cys | 366 | 9.60 |
| 4c | 352 | 7.64 | 5c | 434 | 11.40 | 6c | 338 | 17.85 | 6c+Cys | 344 | 15.90 |
| 4d | 350 | 11.29 | 5d | 437 | 13.70 | 6d | 353 | 9.00 | 6d+Cys | 351 | 7.45 |
| 4e | 354 | 14.85 | 5e | 441 | 10.15 | 6e | 354 | 9.75 | 6e+Cys | 355 | 8.55 |
| 4f | 352 | 13.70 | 5f | 436 | 10.55 | 6f | 352 | 7.10 | 6f+Cys | 351 | 5.70 |
| 4g | 354 | 13.50 | 5g | 437 | 8.10 | 6g | 344 | 9.55 | 6g+Cys | 348 | 10.60 |
| 4h | 355 | 12.60 | 5h | 440 | 18.40 | 6h | 346 | 7.30 | 6h+Cys | 344 | 8.20 |
| 4i | 354 | 4.45 | 5i | 434 | 10.85 | 6i | 347 | 10.80 | 6i+Cys | 347 | 8.30 |
| 4j | 354 | 8.90 | 5j | 436 | 12.10 | 6j | 344 | 12.25 | 6j+Cys | 349 | 13.30 |
| 4k | 354 | 11.95 | 5k | 437 | 9.65 | 6k | 344 | 15.85 | 6k+Cys | 348 | 14.60 |
| 4l | 356 | 12.70 | 5l | 438 | 10.75 | 6l | 348 | 13.20 | 6l+Cys | 349 | 12.10 |
| 4m | 354 | 11.45 | 5m | 437 | 8.80 | 6m | 364 | 15.05 | 6m+Cys | 364 | 15.90 |
| 4n | 353 | 13.45 | 5n | 437 | 12.15 | 6n | 348 | 14.25 | 6n+Cys | 354 | 14.20 |
| 4o | 357 | 14.15 | 5o | 438 | 15.15 | 6o | 345 | 13.35 | 6o+Cys | 359 | 13.10 |
| 4p | 357 | 13.80 | 5p | 437 | 19.25 | 6p | 345 | 12.40 | 6p+Cys | 345 | 11.15 |
| 4q | 359 | 11.90 | 5q | 437 | 17.10 | 6q | 345 | 10.45 | 6q+Cys | 365 | 9.70 |
| 4r | 352 | 8.75 | 5r | 437 | 30.25 | 6r | 342 | 12.15 | 6r+Cys | 359 | 10.55 |
| 4s | 353 | 5.10 | 5s | 436 | 7.55 | 6s | 341 | 9.05 | 6s+Cys | 340 | 8.65 |
| Compd. | λEx/nm | λEm/nm | Hightb | Compd. | λEx/nm | λEm/nm | Hightb | Compd. | λEx/nm | λEm/nm | ΦFa/% |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 5a | 440 | 537 | 214.2 | 6a | 274 | 328 | 67.6 | 6a+Cys | 378 | 468 | 9.06 |
| 5b | 440 | 537 | 200.7 | 6b | 272 | 328 | 171.0 | 6b+Cys | 372 | 472 | —c |
| 5c | 276 | 327 | 167.0 | 6c | 273 | 326 | 182.0 | 6c+Cys | 372 | 470 | —c |
| 5d | 440 | 536 | 213.5 | 6d | 280 | 310 | 28.7 | 6d+Cys | 378 | 470 | 11.36 |
| 5e | 440 | 537 | 191.8 | 6e | 280 | 310 | 33.9 | 6e+Cys | 370 | 467 | 12.56 |
| 5f | 440 | 538 | 149.6 | 6f | 370 | 472 | 66.6 | 6f+Cys | 375 | 468 | 16.32 |
| 5g | 270 | 327 | 75.62 | 6g | 270 | 324 | 60.2 | 6g+Cys | 370 | 465 | —c |
| 5h | 257 | 340 | 209.0 | 6h | 280 | 310 | 36.7 | 6h+Cys | 370 | 468 | 0.18 |
| 5i | 440 | 539 | 83.35 | 6i | 273 | 325 | 72.3 | 6i+Cys | 371 | 468 | —c |
| 5j | 438 | 540 | 135.7 | 6j | 280 | 310 | 33.1 | 6j+Cys | 380 | 469 | 1.52 |
| 5k | 440 | 538 | 119.6 | 6k | 280 | 310 | 29.6 | 6k+Cys | 370 | 471 | 1.50 |
| 5l | 440 | 536 | 167.8 | 6l | 370 | 466 | 48.7 | 6l+Cys | 370 | 468 | 19.25 |
| 5m | 442 | 540 | 97.3 | 6m | 280 | 310 | 34.8 | 6m+Cys | 370 | 459 | —c |
| 5n | 440 | 538 | 85.3 | 6n | 280 | 310 | 32.6 | 6n+Cys | 370 | 468 | 11.89 |
| 5o | 280 | 311 | 36.3 | 6o | 280 | 311 | 34.1 | 6o+Cys | 370 | 470 | 8.26 |
| 5p | 440 | 542 | 105.9 | 6p | 280 | 310 | 35.2 | 6p+Cys | 372 | 466 | 6.71 |
| 5q | 440 | 540 | 161.4 | 6q | 280 | 310 | 35.2 | 6q+Cys | 370 | 470 | 17.89 |
| 5r | 440 | 538 | 98.6 | 6r | 280 | 310 | 36.8 | 6r+Cys | 370 | 462 | —c |
| 5s | 440 | 542 | 35.0 | 6s | 280 | 310 | 36.3 | 6s+Cys | 370 | 309 | 0.13 |
| Compd. | λEx/nm | λEm/nm | Hightb | Compd. | λEx/nm | λEm/nm | Hightb | Compd. | λEx/nm | λEm/nm | ΦFa/% |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 5a | 440 | 537 | 214.2 | 6a | 274 | 328 | 67.6 | 6a+Cys | 378 | 468 | 9.06 |
| 5b | 440 | 537 | 200.7 | 6b | 272 | 328 | 171.0 | 6b+Cys | 372 | 472 | —c |
| 5c | 276 | 327 | 167.0 | 6c | 273 | 326 | 182.0 | 6c+Cys | 372 | 470 | —c |
| 5d | 440 | 536 | 213.5 | 6d | 280 | 310 | 28.7 | 6d+Cys | 378 | 470 | 11.36 |
| 5e | 440 | 537 | 191.8 | 6e | 280 | 310 | 33.9 | 6e+Cys | 370 | 467 | 12.56 |
| 5f | 440 | 538 | 149.6 | 6f | 370 | 472 | 66.6 | 6f+Cys | 375 | 468 | 16.32 |
| 5g | 270 | 327 | 75.62 | 6g | 270 | 324 | 60.2 | 6g+Cys | 370 | 465 | —c |
| 5h | 257 | 340 | 209.0 | 6h | 280 | 310 | 36.7 | 6h+Cys | 370 | 468 | 0.18 |
| 5i | 440 | 539 | 83.35 | 6i | 273 | 325 | 72.3 | 6i+Cys | 371 | 468 | —c |
| 5j | 438 | 540 | 135.7 | 6j | 280 | 310 | 33.1 | 6j+Cys | 380 | 469 | 1.52 |
| 5k | 440 | 538 | 119.6 | 6k | 280 | 310 | 29.6 | 6k+Cys | 370 | 471 | 1.50 |
| 5l | 440 | 536 | 167.8 | 6l | 370 | 466 | 48.7 | 6l+Cys | 370 | 468 | 19.25 |
| 5m | 442 | 540 | 97.3 | 6m | 280 | 310 | 34.8 | 6m+Cys | 370 | 459 | —c |
| 5n | 440 | 538 | 85.3 | 6n | 280 | 310 | 32.6 | 6n+Cys | 370 | 468 | 11.89 |
| 5o | 280 | 311 | 36.3 | 6o | 280 | 311 | 34.1 | 6o+Cys | 370 | 470 | 8.26 |
| 5p | 440 | 542 | 105.9 | 6p | 280 | 310 | 35.2 | 6p+Cys | 372 | 466 | 6.71 |
| 5q | 440 | 540 | 161.4 | 6q | 280 | 310 | 35.2 | 6q+Cys | 370 | 470 | 17.89 |
| 5r | 440 | 538 | 98.6 | 6r | 280 | 310 | 36.8 | 6r+Cys | 370 | 462 | —c |
| 5s | 440 | 542 | 35.0 | 6s | 280 | 310 | 36.3 | 6s+Cys | 370 | 309 | 0.13 |
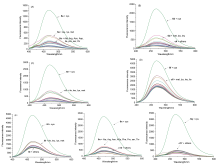

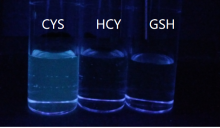
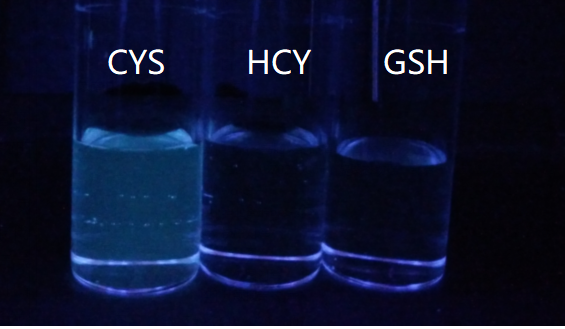
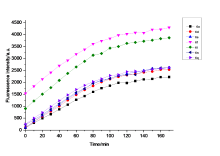



| [1] |
(a) Sun, Y.; Fu, M. L; Bian, M. L; Zhu, Q. Biotechnol Bioeng. 2023, 120, 7.
doi: 10.1002/bit.v120.1 |
|
(b) Ahmed, N.; Zareen, W.; Ye, Y. Chin. Chem. Lett. 2022, 33, 2765.
doi: 10.1016/j.cclet.2021.12.092 |
|
|
(c) Liu, F. R.; Jing, J.; Zhang, X. L. Chin. J. Org. Chem. 2023, 43, 2053 (in Chinese).
doi: 10.6023/cjoc202209005 |
|
|
(刘飞冉, 敬静, 张小玲, 有机化学, 2023, 43, 2053.)
|
|
| [2] |
(a) Kolinska, J.; Grzelakowska, A. Spectrochim. Acta, A Mol. Biomol. Spectrosc. 2021, 262, 120151.
doi: 10.1016/j.saa.2021.120151 |
|
(b) Zhang, Y. L; Shao, X. M; Wang, Y.; Pan, F. C.; Kang, R. X.; Peng, F. F.; Huang, Z. T.; Zhang, W. J; Zhao, W. L. Chem. Commun. 2015, 51, 4245.
doi: 10.1039/C4CC08687B |
|
|
(c) Ge, C. P.; Wang, H.; Zhang, B. X.; Yao, J.; Li, X. M; Feng, W. M; Zhou, P. P.; Wang, Y. W; Fang, J. G Chem. Commun. 2015, 51, 14913.
|
|
| [3] |
(a) Xu, Z. Y.; Zhao, X. F.; Zhou, M.; Zhang, Z. J; Qin, T. Y; Wang, D.; Wang, L.; Peng, X. J.; Liu, B. Sens. Actuators, B 2021, 345, 130367.
doi: 10.1016/j.snb.2021.130367 |
|
(b) Xu, Z. Y.; Si, S. F.; Zhang, Z. J; Tan, H. Y; Qin, T. Y; Wang, Z. L; Wang, D.; Wang, L.; Liu, B. Anal. Chim. Acta 2021, 1176, 338763.
doi: 10.1016/j.aca.2021.338763 |
|
| [4] |
(a) Anbu, S.; Paul, A.; Surendranath, K.; Sidali, A.; Pombeiro, A. J. L. J. Inorg. Biochem. 2021, 220, 111466.
doi: 10.1016/j.jinorgbio.2021.111466 |
|
(b) Dimov, S. M.; Georgiev, N. I.; Asiri, A. M.; Bojinov, V. B J. Fluoresc. 2014, 24, 1621.
|
|
|
(c) Georgiev, N. I.; Dimov, S. M.; Asiri, A. M.; Alamry, K. A.; Obaid, A.Y.; Bojinov, V. B. J. Lumin. 2014, 149, 325.
doi: 10.1016/j.jlumin.2014.01.028 |
|
| [5] |
Qu, L. J.; Yin, C. X; Huo, F. J.; Li, J. F; Chao, J. B; Zhang, Y. B. Sens. Actuators, B 2014, 195, 246.
doi: 10.1016/j.snb.2014.01.026 |
| [6] |
Vasilev, A. A.; Baluschev, S.; Cheshmedzhieva, D.; Ilieva, S.; Castano, O. D.; Vaquero, J. J.; Angelova, S. E.; Landfester, K. Aus. J. Chem. 2015, 68, 1399.
doi: 10.1071/CH15139 |
| [7] |
Girouard, S.; Houle, M. H.; Grandbois, A.; Keillor, J. W.; Michnick, S. W. J. Am. Chem. Soc. 2005, 127, 559.
doi: 10.1021/ja045742x |
| [8] |
Khosravi, A.; Moradian, S.; Gharanjig, K.; Afshar, F. T. J. Chin. Chem. Soc. 2005, 52, 495.
doi: 10.1002/jccs.v52.3 |
| [9] |
Alexiou, M. S.; Tyman, J. H. P. J. Chem. Res. Miniprint 2000, 5, 632.
|
| [10] |
Costi, M. P.; Tondi, D.; Rinaldi, M.; Barlocco, D.; Cignarella, G.; Santi, D. V.; Musiu, C.; Pudu, I.; Vacca, G.; Colla, P. L. Eur. J. Med. Chem. 1996, 31, 1011.
doi: 10.1016/S0223-5234(97)86180-6 |
| [11] |
Cui, L.; Peng, Z. X.; Ji, C. F.; Huang, J. H. Huang, D. T.; Ma, J.; Zhang, S. P.; Qian, X. H.; Xu, Y. F. Chem. Commun. 2014, 50, 1485.
doi: 10.1039/C3CC48304E |
| [12] |
Zhou, J; Fang, C. L; Liu, Y; Zhao, Y; Zhang, N; Liu, X. J.; Wang, F. Y.; Shangguan, D. H. Org. Biomol. Chem. 2015, 13, 3931.
doi: 10.1039/C5OB00302D |
| [13] |
Wang, J. F; Jin, S.; Akay, S.; Wang, B. H. Eur. J. Med. Chem., 2007, 13, 2091.
|
| [14] |
Yuan, D. W; Brown, R. G.; Hepworth, J. D.; Alexiou, M. S.; Tyman, J. H. P. J. Heterocycl. Chem. 2008, 45, 397.
doi: 10.1002/jhet.v45:2 |
| [15] |
Li, X. M; Zheng, Y. J; Tong, H. J; Qian, R.; Zhou, L.; Liu, G. X; Tang, Y.; Li, H.; Lou, K. Y.; Wang, W. Chem.-Eur. J. 2016, 27, 9247.
|
| [1] | 冯康博, 陈炯, 古双喜, 王海峰, 陈芬儿. 全连续流反应技术在药物合成中的新进展(2019~2022)[J]. 有机化学, 2024, 44(2): 378-397. |
| [2] | 李鹏辉, 谢青洋, 万福贤, 张元红, 姜林. 含环丙基的新型取代嘧啶-5-甲酰胺的合成及杀菌活性研究[J]. 有机化学, 2024, 44(2): 650-656. |
| [3] | 邹发凯, 王能中, 姚辉, 王慧, 刘明国, 黄年玉. 1β-/3R-芳基硫代糖的区域与立体选择性合成[J]. 有机化学, 2024, 44(2): 593-604. |
| [4] | 李路瑶, 贺忠文, 张振国, 贾振华, 罗德平. 三芳基碳正离子在有机合成中的应用[J]. 有机化学, 2024, 44(2): 421-437. |
| [5] | 梅青刚, 李清寒. 可见光促进C(3)(杂)芳硫基吲哚化合物的合成研究进展[J]. 有机化学, 2024, 44(2): 398-408. |
| [6] | 赵茜帆, 陈永正, 张世明. 碳基非金属催化剂在有机合成领域的应用及机理研究[J]. 有机化学, 2024, 44(1): 137-147. |
| [7] | 陈珊, 陈志林, 胡琼, 蒙艳双, 黄悦, 陶萍芳, 卢丽如, 黄国保. 含双硫脲基团分子钳在非极性溶剂中识别中性分子[J]. 有机化学, 2024, 44(1): 277-281. |
| [8] | 王化坤, 任晓龙, 宣宜宁. 卤盐催化的α,β-环氧羧酸酯与异氰酸酯[3+2]环加成反应研究[J]. 有机化学, 2024, 44(1): 251-258. |
| [9] | 金玉坤, 任保轶, 梁福顺. 可见光介导的三氟甲基的选择性C-F键断裂及其在偕二氟类化合物合成中的应用[J]. 有机化学, 2024, 44(1): 85-110. |
| [10] | 马翠云, 罗海澜, 张福华, 郭丹, 陈树兴, 王飞. 3-Pyrrolyl BODIPY的绿色生物合成、光物理性质及应用研究[J]. 有机化学, 2024, 44(1): 216-223. |
| [11] | 王博珍, 张婕, 粘春惠, 金茗茗, 孔苗苗, 李物兰, 何文斐, 吴建章. 含有3,4-二氯苯基的酰胺类化合物的合成及抗肿瘤活性研究[J]. 有机化学, 2024, 44(1): 232-241. |
| [12] | 张莹珍, 江丹丹, 李娟华, 王菁菁, 刘昆明, 刘晋彪. 高选择性硒代半胱氨酸荧光探针的构建策略及成像[J]. 有机化学, 2024, 44(1): 41-53. |
| [13] | 于士航, 刘嘉威, 安碧玉, 边庆花, 王敏, 钟江春. 黑腹尼虎天牛接触性信息素的不对称合成[J]. 有机化学, 2024, 44(1): 301-308. |
| [14] | 李阳, 袁锦鼎, 赵頔. 低共熔溶剂1,3-二甲基脲/L-(+)-酒石酸中(E)-2-苯乙烯基喹啉-3-羧酸类衍生物的绿色合成[J]. 有机化学, 2023, 43(9): 3268-3276. |
| [15] | 岁丹丹, 岑南楠, 龚若蕖, 陈阳, 陈文博. 无支持电解质条件下连续流电化学合成三氟甲基化氧化吲哚[J]. 有机化学, 2023, 43(9): 3239-3245. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||