有机化学 ›› 2024, Vol. 44 ›› Issue (9): 2760-2776.DOI: 10.6023/cjoc202405015 上一篇 下一篇
研究论文
荀苗苗a,b, 郭晶晶a,b, 马文兵a, 李宇强a, 袁长春a,b,*( ), 傅凯a,b,*(
), 傅凯a,b,*( )
)
收稿日期:2024-05-13
修回日期:2024-07-17
发布日期:2024-08-16
通讯作者:
袁长春, 傅凯
基金资助:
Miaomiao Xuna,b, Jingjing Guoa,b, Wenbing Maa, Yuqiang Lia, Changchun Yuana,b( ), Kai Fua,b(
), Kai Fua,b( )
)
Received:2024-05-13
Revised:2024-07-17
Published:2024-08-16
Contact:
Changchun Yuan, Kai Fu
Supported by:文章分享

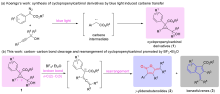
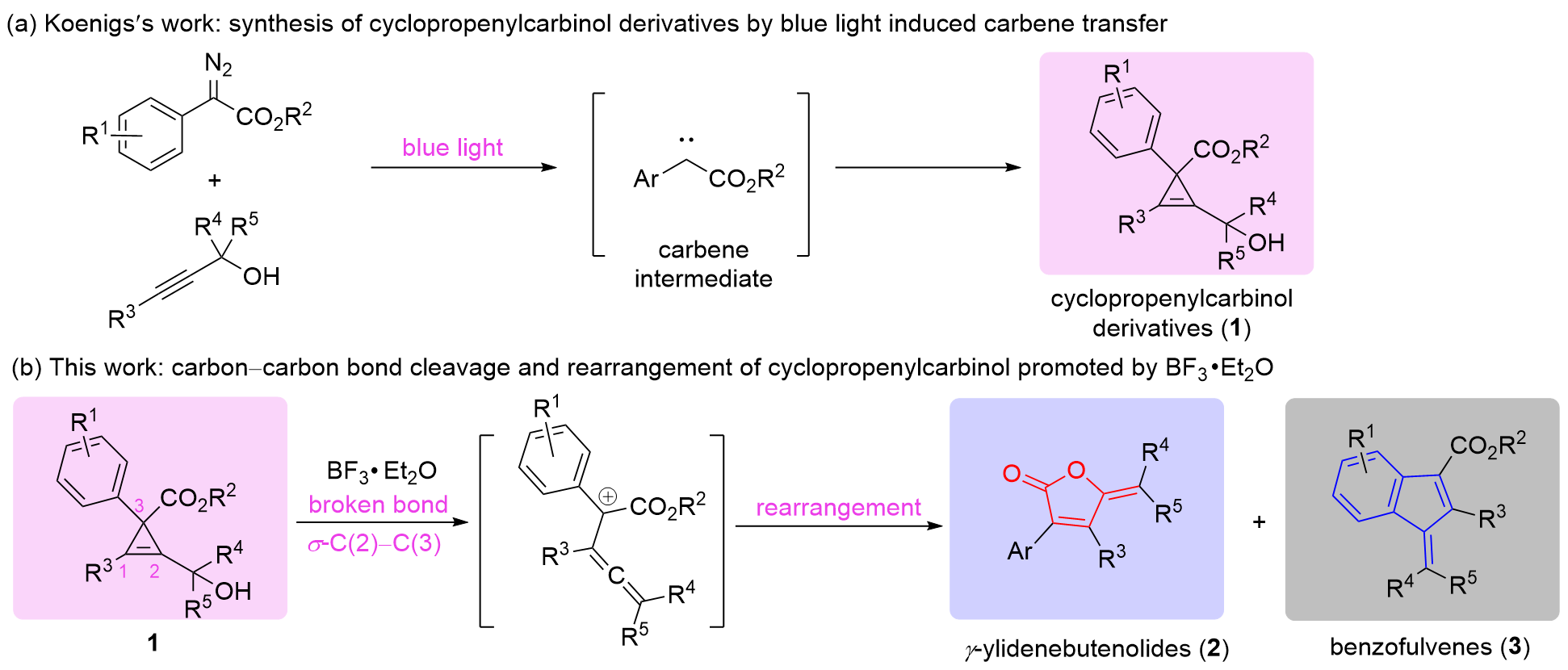
γ-亚乙烯基-丁烯酸内酯和苯并富烯结构单元在活性天然产物和药物化学中都具有重要的意义. 环丙烯基甲醇衍生物在BF3•Et2O的促进下, 脱除羟基后诱导环丙烯C—C断键反应的发生, 并形成联烯碳正离子中间体, 最后经不同的异构重排路径可以“一锅”实现γ-亚乙烯基-丁烯酸内酯和苯并富烯两类化合物的构建. 该合成方法拓展了两类化合物的合成策略, 二者的总收率可达68%~99%, 探究还发现电子效应和反应温度对二者的比例有着较大影响. 此外, 该反应为环丙烯结构的精准C—C键断裂提供了新方法.
荀苗苗, 郭晶晶, 马文兵, 李宇强, 袁长春, 傅凯. BF3•Et2O促进的环丙烯基甲醇衍生物重排反应研究[J]. 有机化学, 2024, 44(9): 2760-2776.
Miaomiao Xun, Jingjing Guo, Wenbing Ma, Yuqiang Li, Changchun Yuan, Kai Fu. Study of BF3•Et2O-Promoted Rearrangement of Cyclopropenylcarbinols[J]. Chinese Journal of Organic Chemistry, 2024, 44(9): 2760-2776.
| Entry | Acid (equiv.) | Solvent | T/℃ | Yield/% | Ratio 2a/3a | ||
|---|---|---|---|---|---|---|---|
| 2a | 3a | Total | |||||
| 1 | CH3SO3H (1.0) | Toluene | r.t. | 36 | 28 | 64 | 1.3/1 |
| 2 | p-TsOH (1.0) | Toluene | r.t. to 100 | 41 | 38 | 79 | 1.1/1 |
| 3 | Cu(OTf)2 (2.0) | Toluene | r.t. to 50 | 40 | 28 | 68 | 1.4/1 |
| 4 | Yb(OTf)3 (2.0) | Toluene | r.t. to 100 | 45 | 37 | 82 | 1.2/1 |
| 5 | In(OTf)3 (1.0) | Toluene | r.t. | 49 | 35 | 84 | 1.4/1 |
| 6 | Hf(OTf)4 (1.0) | Toluene | r.t. | 47 | 34 | 82 | 1.4/1 |
| 7 | TiCl4 (1.0) | Toluene | r.t. | 35 | 37 | 72 | 0.9/1 |
| 8 | FeCl3 (1.0) | Toluene | r.t. | 41 | 38 | 79 | 1.1/1 |
| 9 | AlCl3 (1.0) | Toluene | r.t. | 50 | 30 | 80 | 1.7/1 |
| 10 | BF3•OEt2 (1.0) | Toluene | r.t. | 50 | 37 | 87 | 1.4/1 |
| 11b | BF3•OEt2 (5.0) | Toluene | -78 | — | — | — | — |
| 12 | BF3•OEt2 (5.0) | Toluene | -60 | 59 | 33 | 92 | 1.8/1 |
| 13 | BF3•OEt2 (3.0) | Toluene | -40 | 59 | 33 | 92 | 1.8/1 |
| 14 | BF3•OEt2 (1.5) | Toluene | 0 | 50 | 33 | 83 | 1.5/1 |
| 15 | BF3•OEt2 (1.0) | Toluene | 60 | 51 | 36 | 87 | 1.4/1 |
| 16b | BF3•OEt2 (3.0) | THF | -40 or r.t. | — | — | — | — |
| 17 | BF3•OEt2 (3.0) | THF | 50 | 21 | 19 | 40 | 1.1/1 |
| 18 | BF3•OEt2 (3.0) | DCM | -40 | 53 | 34 | 87 | 1.6/1 |
| 19 | BF3•OEt2 (3.0) | DCE | -35 | 52 | 36 | 88 | 1.4/1 |
| 20 | BF3•OEt2 (3.0) | EA | -40 to r.t. | 50 | 26 | 76 | 1.9/1 |
| 21 | BF3•OEt2 (3.0) | n-Hexane | r.t. | 41 | 38 | 79 | 1.1/1 |
| 22c | BF3•OEt2 (3.0) | Toluene | -40 | 60 | 33 | 93 | 1.8/1 |
| Entry | Acid (equiv.) | Solvent | T/℃ | Yield/% | Ratio 2a/3a | ||
|---|---|---|---|---|---|---|---|
| 2a | 3a | Total | |||||
| 1 | CH3SO3H (1.0) | Toluene | r.t. | 36 | 28 | 64 | 1.3/1 |
| 2 | p-TsOH (1.0) | Toluene | r.t. to 100 | 41 | 38 | 79 | 1.1/1 |
| 3 | Cu(OTf)2 (2.0) | Toluene | r.t. to 50 | 40 | 28 | 68 | 1.4/1 |
| 4 | Yb(OTf)3 (2.0) | Toluene | r.t. to 100 | 45 | 37 | 82 | 1.2/1 |
| 5 | In(OTf)3 (1.0) | Toluene | r.t. | 49 | 35 | 84 | 1.4/1 |
| 6 | Hf(OTf)4 (1.0) | Toluene | r.t. | 47 | 34 | 82 | 1.4/1 |
| 7 | TiCl4 (1.0) | Toluene | r.t. | 35 | 37 | 72 | 0.9/1 |
| 8 | FeCl3 (1.0) | Toluene | r.t. | 41 | 38 | 79 | 1.1/1 |
| 9 | AlCl3 (1.0) | Toluene | r.t. | 50 | 30 | 80 | 1.7/1 |
| 10 | BF3•OEt2 (1.0) | Toluene | r.t. | 50 | 37 | 87 | 1.4/1 |
| 11b | BF3•OEt2 (5.0) | Toluene | -78 | — | — | — | — |
| 12 | BF3•OEt2 (5.0) | Toluene | -60 | 59 | 33 | 92 | 1.8/1 |
| 13 | BF3•OEt2 (3.0) | Toluene | -40 | 59 | 33 | 92 | 1.8/1 |
| 14 | BF3•OEt2 (1.5) | Toluene | 0 | 50 | 33 | 83 | 1.5/1 |
| 15 | BF3•OEt2 (1.0) | Toluene | 60 | 51 | 36 | 87 | 1.4/1 |
| 16b | BF3•OEt2 (3.0) | THF | -40 or r.t. | — | — | — | — |
| 17 | BF3•OEt2 (3.0) | THF | 50 | 21 | 19 | 40 | 1.1/1 |
| 18 | BF3•OEt2 (3.0) | DCM | -40 | 53 | 34 | 87 | 1.6/1 |
| 19 | BF3•OEt2 (3.0) | DCE | -35 | 52 | 36 | 88 | 1.4/1 |
| 20 | BF3•OEt2 (3.0) | EA | -40 to r.t. | 50 | 26 | 76 | 1.9/1 |
| 21 | BF3•OEt2 (3.0) | n-Hexane | r.t. | 41 | 38 | 79 | 1.1/1 |
| 22c | BF3•OEt2 (3.0) | Toluene | -40 | 60 | 33 | 93 | 1.8/1 |
| Entry | Substrate 1 | T/℃ | Product 2 and yield | Product 3 and yield | Total | Ratio 2/3 | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | | -40 | | | 93% | 1.8/1 | ||||||||||||||||||
| 1a | 2a, 60% | 3a, 33% | ||||||||||||||||||||||
| 2 | | -40 | | | 85% | 1.9/1 | ||||||||||||||||||
| 1b | 2a, 56% | 3b, 29% | ||||||||||||||||||||||
| 3 | | -40 | | | 89% | 1.5/1 | ||||||||||||||||||
| 1c | 2a, 54% | 3c, 35% | ||||||||||||||||||||||
| 4 | | -40 | | | 86% | 1.9/1 | ||||||||||||||||||
| 1d | 2d, 56% | 3d, 30% | ||||||||||||||||||||||
| 5b | | -40 | | | 86% | 1/1.5 | ||||||||||||||||||
| 1e | 2e, 34% | 3e, 52% | ||||||||||||||||||||||
| 6 | | -40 | | | 86% | 1.9/1 | ||||||||||||||||||
| 1f | 2f, 56% | 3f, 30% | ||||||||||||||||||||||
| Entry | Substrate 1 | T/℃ | Product 2 and yield | Product 3 and yield | Total | Ratio 2/3 | ||||||||||||||||||
| 7b | | -40 | | | 78% | 1/2.9 | ||||||||||||||||||
| 1g | 2g, 20% | 3g, 58% | ||||||||||||||||||||||
| 8 | | -40 | | | 90% | 1.6/1 | ||||||||||||||||||
| 1h | 2h, 56% | 3h, 34% | ||||||||||||||||||||||
| 9b | | -40 | | | 75% | 1/2.9 | ||||||||||||||||||
| 1i | 2i, 19% | 3i, 56% | ||||||||||||||||||||||
| 10 | | -40 | | | 88% | 2.8/1 | ||||||||||||||||||
| 1j | 2j, 65% | 3j, 23% | ||||||||||||||||||||||
| 11 | | -40 | | | 95% | 1/2.1 | ||||||||||||||||||
| 1k | 2k, 31% | 3k, 64% | ||||||||||||||||||||||
| 12 | | -40 | | | 90% | 1/1.8 | ||||||||||||||||||
| 1l | 2l, 32% | 3l, 58% | ||||||||||||||||||||||
| 13b | | -20 | | | 72% | 1/1.4 | ||||||||||||||||||
| 1m | 2m, 30% | 3m, 42% | ||||||||||||||||||||||
| Entry | Substrate 1 | T/℃ | Product 2 and yield | Product 3 and yield | Total | Ratio 2/3 | ||||||||||||||||||
| 14 | | -40 | | | 68% | 1.1/1 | ||||||||||||||||||
| 1n | 2n, 36% | 3n, 32% | ||||||||||||||||||||||
| 15b,c | | -40 | | | 74% | 1/2.9 | ||||||||||||||||||
| 1o | 2o, 19% | 3o, 55% | ||||||||||||||||||||||
| 16 | | -40 | | | 89% | 1/6.4 | ||||||||||||||||||
| 1p | 2p, 12% | 3p, 77% | ||||||||||||||||||||||
| 17 | | r.t. | | | 94% | 3.7/1 | ||||||||||||||||||
| 1q | 2q, 74% | 3q, 20% | ||||||||||||||||||||||
| 18 | | -40 | | | 83% | 2.8/1 | ||||||||||||||||||
| 1r | 2r, 61% | 3r, 22% | ||||||||||||||||||||||
| 19 | | -40 | | | 85% | 1/1 | ||||||||||||||||||
| 1s | 2s, 42% | 3s, 43% | ||||||||||||||||||||||
| 20 | | -40 | | | 94% | 1/1.2 | ||||||||||||||||||
| 1t | 2t, 43% | 3t, 51% | ||||||||||||||||||||||
| Entry | Substrate 1 | T/℃ | Product 2 and yield | Product 3 and yield | Total | Ratio 2/3 | ||||||||||||||||||
| 21 | | -40 | | | 90% | 2.6/1 | ||||||||||||||||||
| 1u | 2u, 65% | 3u, 25% | ||||||||||||||||||||||
| 22 | | 0 | | | 93% | 2.3/1 | ||||||||||||||||||
| 1v | 2v, 65% | 3v, 28% | ||||||||||||||||||||||
| 23 | | 0 | | | 94% | 4.5/1 | ||||||||||||||||||
| 1w | 2w, 77% | 3w, 17% | ||||||||||||||||||||||
| 24 | | -20 | | | 99% | 2.9/1 | ||||||||||||||||||
| 1x | 2x, 74% | 3x, 25% | ||||||||||||||||||||||
| 25 | | -40 | | | 90% | 1/1.4 | ||||||||||||||||||
| 1y | 2y, 37% | 3y, 53% | ||||||||||||||||||||||
| Entry | Substrate 1 | T/℃ | Product 2 and yield | Product 3 and yield | Total | Ratio 2/3 | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | | -40 | | | 93% | 1.8/1 | ||||||||||||||||||
| 1a | 2a, 60% | 3a, 33% | ||||||||||||||||||||||
| 2 | | -40 | | | 85% | 1.9/1 | ||||||||||||||||||
| 1b | 2a, 56% | 3b, 29% | ||||||||||||||||||||||
| 3 | | -40 | | | 89% | 1.5/1 | ||||||||||||||||||
| 1c | 2a, 54% | 3c, 35% | ||||||||||||||||||||||
| 4 | | -40 | | | 86% | 1.9/1 | ||||||||||||||||||
| 1d | 2d, 56% | 3d, 30% | ||||||||||||||||||||||
| 5b | | -40 | | | 86% | 1/1.5 | ||||||||||||||||||
| 1e | 2e, 34% | 3e, 52% | ||||||||||||||||||||||
| 6 | | -40 | | | 86% | 1.9/1 | ||||||||||||||||||
| 1f | 2f, 56% | 3f, 30% | ||||||||||||||||||||||
| Entry | Substrate 1 | T/℃ | Product 2 and yield | Product 3 and yield | Total | Ratio 2/3 | ||||||||||||||||||
| 7b | | -40 | | | 78% | 1/2.9 | ||||||||||||||||||
| 1g | 2g, 20% | 3g, 58% | ||||||||||||||||||||||
| 8 | | -40 | | | 90% | 1.6/1 | ||||||||||||||||||
| 1h | 2h, 56% | 3h, 34% | ||||||||||||||||||||||
| 9b | | -40 | | | 75% | 1/2.9 | ||||||||||||||||||
| 1i | 2i, 19% | 3i, 56% | ||||||||||||||||||||||
| 10 | | -40 | | | 88% | 2.8/1 | ||||||||||||||||||
| 1j | 2j, 65% | 3j, 23% | ||||||||||||||||||||||
| 11 | | -40 | | | 95% | 1/2.1 | ||||||||||||||||||
| 1k | 2k, 31% | 3k, 64% | ||||||||||||||||||||||
| 12 | | -40 | | | 90% | 1/1.8 | ||||||||||||||||||
| 1l | 2l, 32% | 3l, 58% | ||||||||||||||||||||||
| 13b | | -20 | | | 72% | 1/1.4 | ||||||||||||||||||
| 1m | 2m, 30% | 3m, 42% | ||||||||||||||||||||||
| Entry | Substrate 1 | T/℃ | Product 2 and yield | Product 3 and yield | Total | Ratio 2/3 | ||||||||||||||||||
| 14 | | -40 | | | 68% | 1.1/1 | ||||||||||||||||||
| 1n | 2n, 36% | 3n, 32% | ||||||||||||||||||||||
| 15b,c | | -40 | | | 74% | 1/2.9 | ||||||||||||||||||
| 1o | 2o, 19% | 3o, 55% | ||||||||||||||||||||||
| 16 | | -40 | | | 89% | 1/6.4 | ||||||||||||||||||
| 1p | 2p, 12% | 3p, 77% | ||||||||||||||||||||||
| 17 | | r.t. | | | 94% | 3.7/1 | ||||||||||||||||||
| 1q | 2q, 74% | 3q, 20% | ||||||||||||||||||||||
| 18 | | -40 | | | 83% | 2.8/1 | ||||||||||||||||||
| 1r | 2r, 61% | 3r, 22% | ||||||||||||||||||||||
| 19 | | -40 | | | 85% | 1/1 | ||||||||||||||||||
| 1s | 2s, 42% | 3s, 43% | ||||||||||||||||||||||
| 20 | | -40 | | | 94% | 1/1.2 | ||||||||||||||||||
| 1t | 2t, 43% | 3t, 51% | ||||||||||||||||||||||
| Entry | Substrate 1 | T/℃ | Product 2 and yield | Product 3 and yield | Total | Ratio 2/3 | ||||||||||||||||||
| 21 | | -40 | | | 90% | 2.6/1 | ||||||||||||||||||
| 1u | 2u, 65% | 3u, 25% | ||||||||||||||||||||||
| 22 | | 0 | | | 93% | 2.3/1 | ||||||||||||||||||
| 1v | 2v, 65% | 3v, 28% | ||||||||||||||||||||||
| 23 | | 0 | | | 94% | 4.5/1 | ||||||||||||||||||
| 1w | 2w, 77% | 3w, 17% | ||||||||||||||||||||||
| 24 | | -20 | | | 99% | 2.9/1 | ||||||||||||||||||
| 1x | 2x, 74% | 3x, 25% | ||||||||||||||||||||||
| 25 | | -40 | | | 90% | 1/1.4 | ||||||||||||||||||
| 1y | 2y, 37% | 3y, 53% | ||||||||||||||||||||||
| [1] |
(a) Wang, B.; Perea, M. A.; Sarpong, R. Angew. Chem., Int. Ed. 2020, 59, 18898.
pmid: 32799452 |
|
(b) Leger, P. R.; Kuroda, Y.; Chang, S.; Jurczyk, J.; Sarpong, R. J. Am. Chem. Soc. 2020, 142, 15536.
doi: 10.1021/jacs.0c07316 pmid: 32799452 |
|
|
(c) Hou, S.-H.; Prichina, A. Y.; Dong, G. Angew. Chem., Int. Ed. 2021, 60, 13057.
pmid: 32799452 |
|
|
(d) Zhou, J.; Shi, X.; Zheng, H.; Chen, G.; Zhang, C.; Liu, X.; Cao, H. Org. Lett. 2022, 24, 3238.
pmid: 32799452 |
|
| [2] |
(a) Li, P.; Zhang. X.; Shi. M. Chem. Commun. 2020, 56, 5457.
|
|
(b) Vicente. R. Chem. Rev. 2021, 121, 162.
|
|
| [3] |
Raiguru, B. P.; Nayak, S.; Mishra, D. R.; Das, T.; Mohapatra, S.; Mishra, N. P. Asian J. Org. Chem. 2020, 9, 1088.
doi: 10.1002/ajoc.202000193 |
| [4] |
Seo. S.; Willis, M. C. Org. Lett. 2017, 19, 4556.
|
| [5] |
(a) Uchida, M.; Kusano, G.; Kondo, Y.; Nozoe, S. Heterocycles 1978, 9, 139.
|
|
(b) Yue, G.; Yang, L.; Yuan, C.; Jiang, X.; Liu, B. Org. Lett., 2011, 13, 5406.
|
|
| [6] |
Xia, G.; Zhou, L.; Ma, J.; Wang, Y.; Ding, L.; Zhao, F.; Chen. L.; Qiu, F. Fitoterapia 2015, 103, 143.
|
| [7] |
Frérot, E.; Bagnoud, A.; Vuilleumier, C. Flavour Frag. J. 2002, 17, 218.
|
| [8] |
(a) Miao, S.; Andersen, R. J. J. Org. Chem. 1991, 56, 6275.
pmid: 23030848 |
|
(b) Ortega, M. J.; Zubía, E.; Ocana, M. J.; Naranjo, S.; Salv́a, J. Tetrahedron 2000, 56, 3963.
pmid: 23030848 |
|
|
(c) Sikorska, J.; Parker-Nance, S.; Davies-Coleman, M. T.; Vining, O. B.; Sikora, A. E.; McPhail, K. L. J. Nat. Prod. 2012, 75, 1824.
doi: 10.1021/np300580z pmid: 23030848 |
|
|
(d) Pereira, U. A.; Barbosa, L. C. A.; Maltha, C. R. A.; Demuner, A. J.; Masood, M. A.; Pimenta, A. L. Bioorg. Med. Chem. Lett. 2014, 24, 1052.
pmid: 23030848 |
|
|
(e) Pereira, U. A.; Barbosa, L. C. A.; Maltha, C. R. A.; Demuner, A. J.; Masood, M. A.; Pimenta, A. L. Eur. J. Med. Chem. 2014, 82, 127.
pmid: 23030848 |
|
|
(f) Zhu, T.; Chen, Z.; Liu, P.; Wang, Y.; Xin, Z.; Zhu, W. J. Antibiot. 2014, 67, 315.
pmid: 23030848 |
|
| [9] |
Won, T. H.; Jeon, J.; Kim, S.-H.; Lee, S.-H.; Rho, B. J.; Oh, D.-C.; Oh, K.-B.; Shin, J. J. Nat. Prod. 2012, 75, 2055.
|
| [10] |
Li, L.; Wang, C.-Y.; Huang, H.; Mollo, E.; Cimino, G.; Guo, Y.-W. Helv. Chim. Acta 2008, 91, 111.
|
| [11] |
Wu, Q. H.; Sun, J. D.; Chen, J. W.; Zhang, H. W.; Guo, Y. W.; Wang, H. Mar. Drugs 2018, 16, 320.
|
| [12] |
(a) Walters, M. J.; Blobaum, A. L.; Kingsley, P. J.; Felts, A. S.; Sulikowski, G. A.; Marnett, L. J. Bioorg. Med. Chem. Lett. 2009, 19, 3271.
|
|
(b) Korte, A.; Legros, J.; Bolm, C. Synlett 2004, 2397.
|
|
|
(c) Maguire, A. R.; Papot, S.; Ford, A.; Touhey, S.; O'Connor, R.; Clynes, M. Synlett 2001, 41.
|
|
| [13] |
Felts, A. S.; Siegel, B. S.; Young, S. M.; Moth, C. W.; Lybrand, T. P.; Dannenberg, A. J.; Marnett, L. J.; Subbaramaiah, K. J. Med. Chem. 2008, 51, 4911.
|
| [14] |
(a) Lee, J.; Kim, H.; Lee, T. Gu.; Yang, I.; Won, D. H.; Choi, H.; Nam, S.-J.; Kang, H. J. Nat. Prod. 2014, 77, 1528.
|
|
(b) Shen, Q.; Xu, X.; Liu, C.; Zhao, W.; Xiang, N.; Chen, Y.; Miao, M.; Liu, Z.; Yang, G. Nat. Prod. Res. 2016, 30, 2545.
|
|
| [15] |
(a) Jo, J.; Jeong, M.; Ahn, J.-S.; Akter, J.; Kim, H.-S.; Suh, Y.-G.; Yun, H. J. Org. Chem. 2019, 84, 10953.
|
|
(b) Jeong, M.; Lee, H.; Kim, G.; Jo, J.; Chang, J. W.; Jung, J. H.; Suh, Y.-G.; Yun, H. Eur. J. Org. Chem. 2019, 6714.
|
|
| [16] |
(a) Donslund, B. S.; Nielsen, R. P.; Mønsted, S. M. N.; Jørgensen. K. A. Angew. Chem., Int. Ed. 2016, 55, 11124.
doi: 10.1002/anie.201605079 pmid: 27380751 |
|
(b) Yue, J.-F.; Ran, G.-Y.; Yang, X.-X.; Du, W.; Chen, Y.-C. Org. Chem. Front. 2018, 5, 2676.
pmid: 27380751 |
|
|
(c) Dubois, S.; Rodier, F.; Blanc, R.; Rahmani, R.; Héran, V.; Thibonnet, J.; Commeiras, L.; Parrain, J.-L. Org. Biomol. Chem. 2012, 10, 4712.
pmid: 27380751 |
|
|
(d) Yuan, C.; Du, B.; Deng, H.; Man, Y.; Liu, B. Angew. Chem., Int. Ed. 2017, 56, 637.
pmid: 27380751 |
|
|
(e) Du, B.; Huang, Z.; Wang, X.; Chen, T.; Shen, G.; Fu, S.; Liu, B. Nat. Commun. 2019, 10, 1892.
pmid: 27380751 |
|
| [17] |
(a) Yuan, C.; Zhong, S.; Li, X.; Wang, Y.; Xun, M.; Bai, Y.; Zhu, K. Org. Biomol. Chem. 2018, 16, 7843.
|
|
(b) Li, X.; Wang, Y.; Fu. K.; Hu, Z; Li, Z.; Ma, W.; Xun, M.; Yuan, C. J. Heterocycl. Chem. 2020, 57, 2056.
|
|
| [18] |
(a) Basheer, A.; Mishima, M.; Marek, I. Org. Lett. 2011, 13, 4076.
doi: 10.1021/ol201581c pmid: 21732683 |
|
(b) Mata, S.; López, L. A.; Vicente, R. Angew. Chem., Int. Ed. 2018, 57, 11422.
pmid: 21732683 |
|
| [19] |
Ernouf, G.; Brayer, J.-L.; Meyer, C.; Cossy, J. Beilstein J. Org. Chem. 2019, 15, 333.
|
| [20] |
Smyrnov, V.; Waser, J. Org. Lett. 2023, 25, 6999.
|
| [21] |
(a) Zohar, E.; Marek. I. Org. Lett. 2004, 6, 341.
pmid: 19609999 |
|
(b) Simaan, S.; Masarwa, A.; Zohar, E.; Stanger, A.; Bertus, P.; Marek, I. Chem.-Eur. J. 2009, 15, 8449.
doi: 10.1002/chem.200901074 pmid: 19609999 |
|
| [22] |
He, F.; Koenigs, R. M. Chem. Commun. 2019, 55, 4881.
|
| [23] |
(a) Li, H.; Hao, W. J.; Wang, M.; Qin, X.; Tu, S. J.; Zhou, P.; Li, G. G.; Wang, J. Y.; Jiang, B. Org. Lett. 2018, 20, 4362.
|
|
(b) Meng, S. Y.; Wang, Y. L.; Liu, J.; Zheng, J.; Qian, X.; Wang, Q. R. Org. Lett. 2022, 24, 757.
|
|
|
(c) Chen, Z.; Yu, S.; Zhou, Y.; Li, H.; Qiu, Q.; Li, M.; Wang, Z. Chin. J. Org. Chem. 2023, 43, 3107 (in Chinese).
|
|
|
(陈祖佳, 宇世伟, 周永军, 李焕清, 邱琪雯, 李妙欣, 汪朝阳, 有机化学, 2023, 43, 3107.)
doi: 10.6023/cjoc202303006 |
|
| [24] |
Muthusamy, S.; Sivaguru, M. Org. Lett. 2014, 16, 4248.
|
| [1] | 张书林, 张周, 孙萌. 银催化环丙烯酮与醇的C—C键断裂加成反应[J]. 有机化学, 2024, 44(7): 2233-2240. |
| [2] | 张泽鹏, 郜云鹏, 陈树峰, 王剑波. 过渡金属催化的环丙烯聚合反应研究[J]. 有机化学, 2021, 41(5): 1888-1896. |
| [3] | 刘思展, 崔明月, 王博文, 胡春梅, 郑莹莹, 李晶, 徐学涛, 王震, 王少华. 三乙胺促进的环丙烯酮和α-卤代异羟肟酸酯的环化反应合成多取代6H-1,3-噁嗪-6-酮[J]. 有机化学, 2021, 41(4): 1622-1630. |
| [4] | 史传星, 冯陈国, 陈雅丽, 张曙盛, 林国强. 苯并富烯合成方法研究进展[J]. 有机化学, 2020, 40(4): 817-830. |
| [5] | 许露露, 叶倩雯, 程冬萍, 李小年, 许孝良. N-溴代丁二酰亚胺促进的环丙烯羧酸酯的区域选择性开环反应[J]. 有机化学, 2019, 39(9): 2645-2649. |
| [6] | 陆文兴,颜朝国,习国旺,许文琴. 二苯并富烯紫外光谱的取代基效应[J]. 有机化学, 1995, 15(5): 525-529. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||