Chinese Journal of Organic Chemistry ›› 2021, Vol. 41 ›› Issue (6): 2228-2248.DOI: 10.6023/cjoc202010039 Previous Articles Next Articles
REVIEWS
底慧明( ), 刘云婷, 马艳榕, 杨鑫悦, 金辉*(
), 刘云婷, 马艳榕, 杨鑫悦, 金辉*( ), 张立新*(
), 张立新*( )
)
收稿日期:2020-10-28
修回日期:2020-11-18
发布日期:2021-02-22
通讯作者:
金辉, 张立新
基金资助:
Huiming Di( ), Yunting Liu, Yanrong Ma, Xinyue Yang, Hui Jin(
), Yunting Liu, Yanrong Ma, Xinyue Yang, Hui Jin( ), Lixin Zhang(
), Lixin Zhang( )
)
Received:2020-10-28
Revised:2020-11-18
Published:2021-02-22
Contact:
Hui Jin, Lixin Zhang
Supported by:Share
Huiming Di, Yunting Liu, Yanrong Ma, Xinyue Yang, Hui Jin, Lixin Zhang. Recent Advances in Organocatalytic Asymmetric Synthesis of 3,4-Dihydropyran-2-ones and 3,4-Dihydropyridin-2-ones[J]. Chinese Journal of Organic Chemistry, 2021, 41(6): 2228-2248.

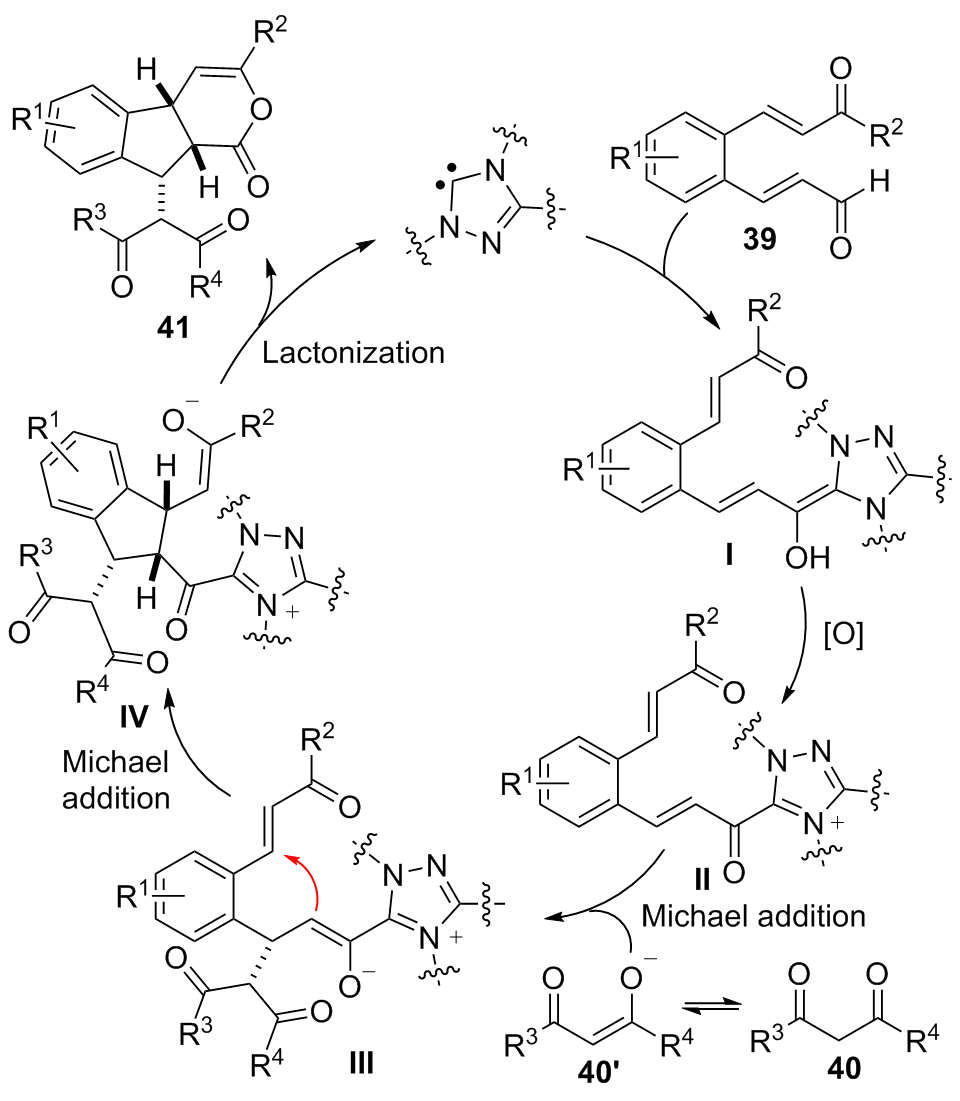


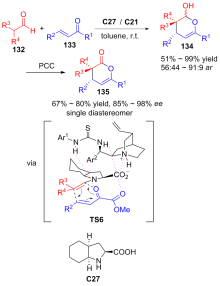
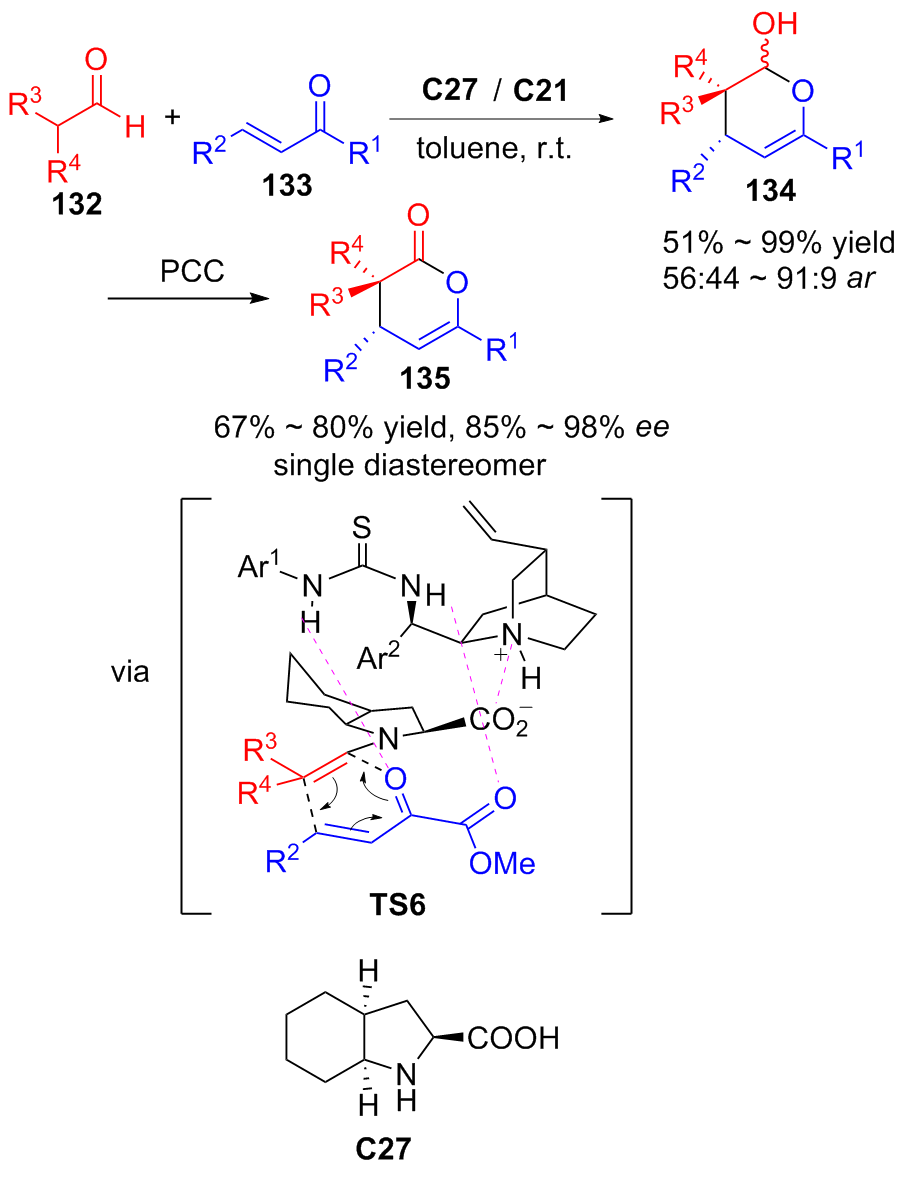
| [1] |
(a) Halliwell, B. Biochem. J. 2007, 401,1.
doi: 10.1042/BJ20061131 pmid: 17201405 |
|
(b) Robert, S.; Bertolla, C.; Masereel, B.; Dogné, J.-M.; Pochet, L. J. Med. Chem. 2008, 51,3077.
doi: 10.1021/jm8002697 pmid: 17201405 |
|
|
(c) Yang, J.; Liu, G.-Y.; Dai, F.; Cao, X.-Y.; Kang, Y.; Hu, L.-M.; Jin, X.-L. Bioorg. Med. Chem. Lett. 2011, 21,6420.
doi: 10.1016/j.bmcl.2011.08.090 pmid: 17201405 |
|
|
(d) Nantermet, P. G.; Barrow, J. C.; Selnick, H. G.; Homnick, C. F.; Freidinger, R. M.; Chang, R. S. L.; O’Malley, S. S.; Reiss, D. R.; Broten, T. P.; Ransom, R. W.; Pettibone, D. J.; Olah, T.; Forray, C. Bioorg. Med. Chem. Lett. 2000, 10,1625.
pmid: 17201405 |
|
|
(e) Goodman, K. B.; Cui, H.; Dowdell, S. E.; Gaitanopoulos, D. E.; Ivy, R. L.; Sehon, C. A.; Stavenger, R. A.; Wang, G. Z.; Viet, A. Q.; Xu, W.; Ye, G.; Semus, S. F.; Evans, C.; Fries, H. E.; Jolivette, L. J.; Kirkpatrick, R. B.; Dul, E.; Khandekar, S. S.; Yi, T.; Jung, D. K.; Wright, L. L.; Smith, G. K.; Behm, D. J.; Bentley, R.; Doe, C. P.; Hu, E.; Lee, D. J. Med. Chem. 2007, 50,6.
pmid: 17201405 |
|
| [2] |
(a) Ying, A.; Wu, C.; Fu, Y.; Ren, S.; Liang, H. Chin. J. Org. Chem. 2012, 32,1587(in Chinese).
doi: 10.6023/cjoc201203009 pmid: 32728081 |
|
( 应安国, 武承林, 付永前, 任世斌, 梁华定, 有机化学, 2012, 32,1587.)
doi: 10.6023/cjoc201203009 pmid: 32728081 |
|
|
(b) Xiang, S.-H.; Tan, B. Nat. Commun. 2020, 11,3786.
doi: 10.1038/s41467-020-17580-z pmid: 32728081 |
|
|
(c) Lassaletta, J. M. Nat. Commun. 2020, 11,3787.
doi: 10.1038/s41467-020-17600-y pmid: 32728081 |
|
|
(d) Zhang, M.-M.; Luo, Y.-Y.; Lu, L.-Q.; Xiao, W.-J. Acta Chim. Sinica 2018, 76,838(in Chinese).
doi: 10.6023/A18060237 pmid: 32728081 |
|
|
( 张毛毛, 骆元元, 陆良秋, 肖文精, 化学学报, 2018, 76,838.)
pmid: 32728081 |
|
| [3] |
(a) Dalko, P. I. Comprehensive Enantioselective Organocata-lysis: Catalysts, Reactions, and Applications, Wiley, New York, 2013.
|
|
(b) Chen, X.-K.; Wang, H.-L.; Jin, Z.-C.; Chi, Y. R. Chin. J. Chem. 2020, 38,1167.
doi: 10.1002/cjoc.v38.10 |
|
| [4] |
(a) Grondal, C.; Jeanty, M.; Enders, D. Nat. Chem. 2010, 2,167.
doi: 10.1038/nchem.539 |
|
(b) Chanda, T.; Zhao, J. C. -G. Adv. Synth. Catal. 2018, 360,2.
doi: 10.1002/adsc.v360.1 |
|
|
(c) Lin, Y. ; D., D-M. Chin. J. Org. Chem. 2020, 40,3214(in Chinese).
doi: 10.6023/cjoc202005065 |
|
|
( 林晔, 杜大明, 有机化学, 2020, 40,3214.)
doi: 10.6023/cjoc202005065 |
|
|
(d) Sun, L.-H.; Liang, Z.-Q.; Ye, S. Acta Chim. Sinica 2014, 72,841(in Chinese).
doi: 10.6023/A14040334 |
|
|
( 孙利辉, 梁志钦, 叶松, 化学学报, 2014, 72,841.)
|
|
|
(e) Zheng, Y.; Xie, Z.-Z.; Chen, K.; Xiang, H.-Y.; Yang, H. Chin. J. Org. Chem. 2021, 41,1(in Chinese).
doi: 10.6023/cjoc202008037 |
|
|
( 郑雨, 谢珍珍, 陈凯, 向皞月, 阳华, 有机化学, 2021, 41,1.)
doi: 10.6023/cjoc202008037 |
|
| [5] |
Belmessieri, D.; Morrill, L. C.; Simal, C.; Slawin, A. M.; Smith, A. D. J. Am. Chem. Soc. 2011, 133,2714.
doi: 10.1021/ja109975c pmid: 21302961 |
| [6] |
(a) Oehlrich, D.; Berthelot, D. J. -C.; Gijsen, H. J. M. J. Med. Chem. 2011, 54,699.
doi: 10.1021/jm1010513 pmid: 22342629 |
|
(b) Chen, J.; Lu, M.-M.; Liu, B.; Chen, Z.; Li, Q.-B.; Tao, L.-J.; Hu, G.-Y. Bioorg. Med. Chem. Lett. 2012, 22,2300.
doi: 10.1016/j.bmcl.2012.01.073 pmid: 22342629 |
|
|
(c) Li, P.; Liu, L.-J.; Liu, J.-T. Org. Biomol. Chem. 2011, 9,74.
doi: 10.1039/C0OB00699H pmid: 22342629 |
|
|
(d) Yi, H.; Song, L. P.; Wang, W.; Liu, J. N.; Zhu, S. Z.; Deng, H. M.; Shao, M. Chem. Commun. 2010, 46,6941.
doi: 10.1039/c0cc01815e pmid: 22342629 |
|
| [7] |
Morrill, L. C.; Douglas, J.; Lebl, T.; Slawin, A. M.; Fox, D. J.; Smith, A. D. Chem. Sci. 2013, 4,4146.
doi: 10.1039/c3sc51791h |
| [8] |
Simal, C.; Lebl, T.; Slawin, A. M. Z.; Smith, A. D. Angew. Chem., Int. Ed. 2012, 51,3653.
doi: 10.1002/anie.v51.15 |
| [9] |
Yeh, P. P.; Daniels, D. S. B.; Fallan, C.; Gould, E.; Simal, C.; Taylor, J. E.; Smith, A. D. Org. Biomol. Chem. 2015, 13,2177.
doi: 10.1039/C4OB02408G |
| [10] |
Stark, D. G.; Young, C. M.; O’Riordan, T. J. C.; Slawin, A. M. Z.; Smith, A. D. Org. Biomol. Chem. 2016, 14,8068.
doi: 10.1039/C6OB01473A |
| [11] |
Stark, D. G.; Morrill, L. C.; Cordes, D. B.; Slawin, A. M. Z.; O’Riordan, T. J. C.; Smith, A. D. Chem. Asian J. 2016, 11,395.
doi: 10.1002/asia.v11.3 |
| [12] |
Young, C. M.; Stark, D. G.; West, T. H.; Taylor, J. E.; Smith, A. D. Angew. Chem., Int. Ed. 2016, 55,14394.
doi: 10.1002/anie.v55.46 |
| [13] |
Lee, J. W.; Mayer-Gall, T.; Opwis, K.; Song, C. E.; Gutmann, J. S.; List, B. Science 2013, 341,1225.
doi: 10.1126/science.1242196 |
| [14] |
Wang, S.; Izquierdo, J.; Rodríguez-Escrich, C.; Pericàs, M. A. ACS Catal. 2017, 7,2780.
doi: 10.1021/acscatal.7b00360 |
| [15] |
Zhang, Y.-C.; Geng, R.-L.; Song, J.; Gong, L.-Z. Org. Lett. 2020, 22,2261.
doi: 10.1021/acs.orglett.0c00461 |
| [16] |
Liu, H.; Slawin, A. M. Z.; Smith, A. D. Org. Lett. 2020, 22,1301.
doi: 10.1021/acs.orglett.9b04615 |
| [17] |
(a) Nozawa, K.; Nakajima, S. J. Nat. Prod. 1979, 42,374.
doi: 10.1021/np50004a004 |
|
(b) Koizumi, Y.; Arai, M.; Tomoda, H.; Omura, S. Biochim. Biophys. Acta, Mol. Cell Res. 2004,1693 , 47.
|
|
|
(c) He, R.; Ding, C.; Maruoka, K. Angew. Chem., Int. Ed. 2009, 48,4559.
doi: 10.1002/anie.v48:25 |
|
|
(d) Guo, C.; Song, J.; Luo, S.-W.; Gong, L.-Z. Angew. Chem., Int. Ed. 2010, 49,5558.
doi: 10.1002/anie.201002108 |
|
|
(e) Yao, J.; Wei, X.; Lu, Y. Biochem. Biophys. Res. Commun. 2016, 473,867.
doi: 10.1016/j.bbrc.2016.03.141 |
|
| [18] |
(a) Flanigan, D. M.; Romanov-Michailidis, F.; White, N. A.; Rovis, T. Chem. Rev. 2015, 115,9307.
doi: 10.1021/acs.chemrev.5b00060 pmid: 25992594 |
|
(b) Chen, X. -.; Gao, Z. -.; Ye, S. Acc. Chem. Res. 2020, 53,690.
doi: 10.1021/acs.accounts.9b00635 pmid: 25992594 |
|
|
(c) Wang, A.; Xiao, Y. -.; Zhou, Y.; Xu, J. -.; Liu, H. Chin. J. Org. Chem. 2017, 37,2590(in Chinese).
pmid: 25992594 |
|
|
( 王翱, 肖永龙, 周宇, 徐进宜, 柳红, 有机化学, 2017, 37,2590.)
doi: 10.6023/cjoc201702041 pmid: 25992594 |
|
| [19] |
He, M.; Uc, G. J.; Bode, J. W. J. Am. Chem. Soc. 2006, 128,15088.
doi: 10.1021/ja066380r |
| [20] |
Wang, D.-L.; Liang, Z.-Q.; Chen, K.-Q.; Sun, D.-Q.; Ye, S. J. Org. Chem. 2015, 80,5900.
doi: 10.1021/acs.joc.5b00232 |
| [21] |
Davies, A. T.; Pickett, P. M.; Slawin, A. M. Z.; Smith, A. D. ACS Catal. 2014, 4,2696.
doi: 10.1021/cs500667g |
| [22] |
(a) Ulmschneider, S.; Muller-Vieira, U.; Klein, C. D.; Antes, I.; Lengauer, T.; Hartmann, R. W. J. Med. Chem. 2005, 48,1563.
pmid: 18667309 |
|
(b) Hudson, S.; Kiankarimi, M.; Eccles, W.; Mostofi, Y. S.; Genicot, M. J.; Dwight, W.; Fleck, B. A.; Gogas, K.; Wade, W. S. Bioorg. Med. Chem. Lett. 2008, 18,4495.
doi: 10.1016/j.bmcl.2008.07.050 pmid: 18667309 |
|
|
(c) Camps, P.; Formosa, X.; Galdeano, C.; Gomez, T.; Munoz-Tor- rero, D.; Scarpellini, M.; Viayna, E.; Badia, A.; Clos, M. V.; Camins, A.; Pallas, M.; Bartolini, M.; Mancini, F.; Andrisano, V.; Estelrich, J.; Lizondo, M.; Bidon-Chanal, A.; Luque, F. J. J. Med. Chem. 2008, 51,3588.
doi: 10.1021/jm8001313 pmid: 18667309 |
|
| [23] |
Biswas, A.; Sarkar, S. D.; Fröhlich, R.; Studer, A. Org. Lett. 2011, 13,4966.
doi: 10.1021/ol202108a pmid: 21863845 |
| [24] |
Mukherjee, S.; Ghosh, A.; Marelli, U. K.; Biju, A. T. Org. Lett. 2018, 20,2952.
doi: 10.1021/acs.orglett.8b00998 pmid: 29722983 |
| [25] |
Hao, L.; Chen, S.; Xu, J.; Tiwari, B.; Fu, Z., Li, T.; Chi, Y. R. Org. Lett. 2013, 15,4956.
doi: 10.1021/ol4021805 |
| [26] |
Gharpure, S. J.; Vishwakarma, D. S. Eur. J. Org. Chem. 2020,6887.
|
| [27] |
Lee, A.; Younai, A.; Price, C. K.; Izquierdo, J.; Mishra, R. K.; Scheidt, K. A. J. Am. Chem. Soc. 2014, 136,10589.
doi: 10.1021/ja505880r |
| [28] |
(a) Lv, H.; Zhang, Y.-R.; Huang, X.-L.; Ye, S. Adv. Synth. Catal. 2008, 350,2715.
doi: 10.1002/adsc.200800532 |
|
(b) Huang, X.-L.; He, L.; Shao, P.-L.; Ye, S. Angew. Chem., Int. Ed. 2009, 48,192.
|
|
|
(c) Shao, P.-L.; Chen, X.-Y.; Ye, S. Angew. Chem., Int. Ed. 2010, 49,8412.
doi: 10.1002/anie.201003532 |
|
|
(d) Shao, P.-L.; Chen, X.-Y.; Sun, L.-H.; Ye, S. Tetrahedron Lett. 2010, 51,2316.
doi: 10.1016/j.tetlet.2010.02.122 |
|
|
(e) Zhang, H.-M.; Gao, Z.-H.; Ye, S. Org. Lett. 2014, 16,3079.
doi: 10.1021/ol501205v |
|
| [29] |
Lv, H.; Chen, X.-Y.; Sun, L.; Ye, S. J. Org. Chem. 2010, 75,6973.
doi: 10.1021/jo101318u |
| [30] |
Jian, T.-Y.; Chen, X.-Y.; Sun, L.-H.; Ye, S. Org. Biomol. Chem. 2013, 11,158.
doi: 10.1039/C2OB26804C |
| [31] |
Wang, C.-Y.; Wang, Z.-Y.; Yang, J.; Shi, S.-H.; Hui, X.-P. Org. Lett. 2020, 22,4440.
doi: 10.1021/acs.orglett.0c01447 |
| [32] |
(a) Kaeobamrung, J.; Mahatthananchai, J.; Zheng, P.; Bode, J. W. J. Am. Chem. Soc. 2010, 132,8810.
doi: 10.1021/ja103631u pmid: 20550127 |
|
(b) Ryan, S. J.; Candish, L.; Lupton, D. W. J. Am. Chem. Soc. 2009, 131,14176.
doi: 10.1021/ja905501z pmid: 20550127 |
|
| [33] |
(a) De, Sarkar, S.; Studer, A. Angew. Chem., Int. Ed. 2010, 49,9266.
doi: 10.1002/anie.201004593 |
|
(b) Rong, Z.-Q.; Jia, M.-Q.; You, S.-L. Org. Lett. 2011, 13,4080.
doi: 10.1021/ol201595f |
|
| [34] |
Kravina, A. G.; Mahatthananchai, J.; Bode, J. W. Angew. Chem., Int. Ed. 2012, 51,9433.
doi: 10.1002/anie.201204145 |
| [35] |
(a) Ball-Jones, N. R.; Badillo, J. J.; Franz, A. K. Org. Biomol. Chem. 2012, 10,5165.
doi: 10.1039/c2ob25184a pmid: 22581310 |
|
(b) Cheng, D.; Ishihara, Y.; Tan, B.; Barbas, III, C. F. ACS. Catal. 2014, 4,743.
doi: 10.1021/cs401172r pmid: 22581310 |
|
|
(c) Pavlovska, T. L.; Redkin, R. G.; Lipson, V. V.; Atamanuk, D. V. Mol. Diversity 2016, 20,299.
doi: 10.1007/s11030-015-9629-8 pmid: 22581310 |
|
|
(d) Yu, B.; Cai, Z.; Wang, S.; Liu, H. Chin. J. Org. Chem. 2017, 37,1952(in Chinese).
doi: 10.6023/cjoc201704004 pmid: 22581310 |
|
|
( 余斌, 蔡祖恽, 王帅, 刘宏民, 有机化学, 2017, 37,1952.)
doi: 10.6023/cjoc201704004 pmid: 22581310 |
|
| [36] |
Xie, D.; Yang, L.; Lin, Y.; Zhang, Z.; Chen, D.; Zeng, X.; Zhong, G. Org. Lett. 2015, 17,2318.
doi: 10.1021/acs.orglett.5b00726 |
| [37] |
Zhao, L.-L.; Li, X.-S., Cao, L.-L.; Zhang, R.; Shi, X.-Q.; Qi, J. Chem. Commun. 2017, 53,5985.
doi: 10.1039/C7CC02753B |
| [38] |
Piera, J.; Bäckvall, J.-E. Angew. Chem., Int. Ed. 2008, 47,3506.
doi: 10.1002/(ISSN)1521-3773 |
| [39] |
Xie, D.; Shen, D.; Chen, Q.; Zhou, J.; Zeng, X.; Zhong, G. J. Org. Chem. 2016, 81,6136.
doi: 10.1021/acs.joc.6b01152 |
| [40] |
Axelsson, A.; Hammarvid, E.; Ta, L.; Sundén, H. Chem. Commun. 2016, 52,11571.
doi: 10.1039/C6CC06060A |
| [41] |
Sun, F.-G.; Sun, L.-H.; Ye, S. Adv. Synth. Catal. 2011, 353,3134.
doi: 10.1002/adsc.v353.17 |
| [42] |
Zhang, H.; Jia, W.; Liang, Z.; Ye, S. Asian J. Org. Chem. 2014, 3,462.
doi: 10.1002/ajoc.v3.4 |
| [43] |
Gao, Z.-H.; Chen, X.-Y.; Zhang, H.-M.; Ye, S. Chem. Commun. 2015, 51,12040.
doi: 10.1039/C5CC04593B |
| [44] |
Chen, K.; Gao, Z.; Ye, S. Angew. Chem., Int. Ed. 2019, 58,1183.
doi: 10.1002/anie.v58.4 |
| [45] |
Yan, J.; Song, Z.; Zhao, C.; Shi, K.; Yang, L.; Zhong, G. Org. Lett. 2020, 22,3329.
doi: 10.1021/acs.orglett.0c00699 |
| [46] |
Cheng, J.; Huang, Z.; Chi, Y. R. Angew. Chem., Int. Ed. 2013, 52,8592.
doi: 10.1002/anie.201303247 |
| [47] |
Zhang, Z.; Zeng, X.; Xie, D.; Chen, D.; Ding, L.; Wang, A.; Zhong, G. Org. Lett. 2015, 17,5052.
doi: 10.1021/acs.orglett.5b02527 |
| [48] |
Chen, X.-Y.; Gao, Z.-H.; Song, C.-Y.; Zhang, C.-L.; Wang, Z.-X.; Ye, S. Angew. Chem., Int. Ed. 2014, 53,11611.
doi: 10.1002/anie.201407469 |
| [49] |
Que, Y.; Lu, Y.; Wang, W.; Wang, Y.; Wang, H.; Yu, C.; Yao, C. Chem. Asian J. 2016, 11,678.
doi: 10.1002/asia.201501353 |
| [50] |
(a) Okino, T.; Hoashi, Y.; Takemoto, Y. J. Am. Chem. Soc. 2003, 125,12672.
doi: 10.1021/ja036972z |
|
(b) Okino, T.; Hoashi, Y.; Furukawa, T.; Xu, X.; Takemoto, Y. J. Am. Chem. Soc. 2005, 127,119.
doi: 10.1021/ja044370p |
|
|
(c) Connon, S. J. Chem Commun. 2008, 22,2499.
|
|
| [51] |
Zhang, M. L.; Wu, Z. J.; Zhao, J. Q.; Luo, Y.; Xu, X. Y.; Zhang, X. M.; Yuan, W. C. Org. Lett. 2016, 18,5110.
doi: 10.1021/acs.orglett.6b02558 |
| [52] |
(a) Burke, H. M.; McSweeney, L.; Scanlan, E. M. Nat. Commun. 2017, 8,15655.
doi: 10.1038/ncomms15655 pmid: 27340449 |
|
(b) Bahlinger, A.; Fritz, S. P.; Wennemers, H. Angew. Chem., Int. Ed. 2014, 53,8779.
doi: 10.1002/anie.201310532 pmid: 27340449 |
|
|
(c) Schäberle, T. F. Beilstein J. Org. Chem. 2016, 12,571.
doi: 10.3762/bjoc.12.56 pmid: 27340449 |
|
| [53] |
Jin, H.; Lee, J.; Shi, H.; Lee, J. Y.; Yoo, E. J.; Song, C. E.; Ryu, D. H. Org. Lett. 2018, 20,1584.
doi: 10.1021/acs.orglett.8b00331 |
| [54] |
Cui, L. Y.; Lv, D.; Wang, Y. M.; Fan, Z. J.; Li, Z. M.; Zhou, Z. H. J. Org. Chem. 2018, 83,4221.
doi: 10.1021/acs.joc.8b00234 |
| [55] |
Zhang, S.; Lv, M.; Yin, S.; Li, N.; Zhang, J.; Wang, X. Adv. Synth. Catal. 2016, 358,143.
doi: 10.1002/adsc.v358.1 |
| [56] |
Zhang, Z.-P.; Chen, L.; Li, X.; Cheng, J.-P. J. Org. Chem. 2018, 83,2714.
doi: 10.1021/acs.joc.7b03177 |
| [57] |
Xian, J.; Chen, L.; Ye, L.; Sun, Y.; Shi, Z.; Zhao, Z.; Li, X. Tetrahedron 2019, 75,2350.
doi: 10.1016/j.tet.2019.03.007 |
| [58] |
Chen, L.; Zhang, X.; Shi, K.-J.; Leng, H.-J.; Li, Q.-Z.; Liu, Y.; Li, J.-L. J. Org. Chem. 2020, 85,9454.
doi: 10.1021/acs.joc.0c00957 |
| [59] |
(a) GrAger, H.; Wilken, J. Angew. Chem., Int. Ed. 2001, 40,529.
doi: 10.1002/1521-3773(20010202)40:3【-逻*辑*与-】#x00026;lt;【-逻*辑*与-】#x00026;gt;1.0.CO;2-A |
|
(b) List, B. Tetrahedron 2002, 58,5573.
doi: 10.1016/S0040-4020(02)00516-1 |
|
|
(c) Marigo, M.; Wabnitz, T. C.; Fielenbach, D.; Jørgensen, K. A. Angew. Chem., Int. Ed. 2005, 44,794.
doi: 10.1002/anie.v44:5 |
|
|
(d) Hayashi, Y.; Gotoh, H.; Hayashi, T.; Shoji, M. Angew. Chem., Int. Ed. 2005, 44,4212.
doi: 10.1002/(ISSN)1521-3773 |
|
|
(e) Zhao, C.; Feng, Z.; Xu, G.; Gao, A.; Chen, J.; Wang, Z.; Xu, P. Angew. Chem., Int. Ed. 2020, 59,3058.
doi: 10.1002/anie.v59.8 |
|
| [60] |
Juhl, K.; Jørgensen, K. A. Angew. Chem., Int. Ed. 2003, 42,1498.
doi: 10.1002/anie.200250652 |
| [61] |
Wang, J.; Yu, F.; Zhang, X.; Ma, D. Org. Lett. 2008. 10,2561.
doi: 10.1021/ol800835m |
| [62] |
Sinha, D.; Perera, S.; Zhao, J. C. G. Chem.-Eur. J. 2013, 19,6976.
doi: 10.1002/chem.v19.22 |
| [63] |
Kano, T.; Maruyama, H.; Homma, C.; Maruoka, K. Chem. Commun. 2018, 54,3496.
doi: 10.1039/C8CC01443D |
| [64] |
Kelley, A. M.; Haywood, R. D.; White, J. C.; Petersen, K. S. ChemistrySelect 2020, 5,3018.
doi: 10.1002/slct.v5.10 |
| [65] |
Shirakawa, S.; Maruoka, K. Angew. Chem. Int. Ed. 2013, 52,4312.
doi: 10.1002/anie.201206835 |
| [66] |
Destro, D.; Bottinelli, C.; Ferrari, L.; Albanese, D. C. M.; Bencivenni, G.; Gillick-Healy, M. W.; Adamo, M. F. A. J. Org. Chem. 2020, 85,5183.
doi: 10.1021/acs.joc.9b03216 |
| [1] | Shuang Yang, Xinqiang Fang. Kinetic Resolutions Enabled by N-Heterocyclic Carbene Catalysis: An Update [J]. Chinese Journal of Organic Chemistry, 2024, 44(2): 448-480. |
| [2] | Wanting Chen, Xiongwei Zhong, Jiale Xing, Changshu Wu, Yang Gao. Progress in Asymmetric Catalytic Synthesis of C—N Axis Chiral Compounds [J]. Chinese Journal of Organic Chemistry, 2024, 44(2): 349-377. |
| [3] | Quanbin Jiang. Progress in Synthesis of Axially Chiral Compounds through aza-Vinylidene o-Quinone Methide Intermediates [J]. Chinese Journal of Organic Chemistry, 2024, 44(1): 159-172. |
| [4] | Chun-Xia Cheng, Lu-Ping Wu, Feng Sha, Xin-Yan Wu. Enantioselective Vinylogous Allylic Alkylation of Coumarins with Morita-Baylis-Hillman Carbonates Catalyzed by Chiral Phosphine-Amide [J]. Chinese Journal of Organic Chemistry, 2023, 43(9): 3188-3195. |
| [5] | Yiwen Quan, Xinhui Jiang, Wenjun Li, Jian Wang. Access to α-Vinyl β-Alkynyl Enals via an Organocatalytic Deconjugation-Aldol Condensation Sequence [J]. Chinese Journal of Organic Chemistry, 2023, 43(6): 2120-2125. |
| [6] | Cheng Luo, Yanli Yin, Zhiyong Jiang. Recent Advances in Asymmetric Synthesis of P-Chiral Phosphine Oxides [J]. Chinese Journal of Organic Chemistry, 2023, 43(6): 1963-1976. |
| [7] | Ke Jing, Panke Zhang, Senmiao Xu. Application of 1,4-Azaborines in Organic and Transition Metal Catalysis [J]. Chinese Journal of Organic Chemistry, 2023, 43(5): 1742-1750. |
| [8] | Xinyu Zhang, Huihui Geng, Shilei Zhang, Wei Wang, Xiaobei Chen. A Method for the Synthesis of Deuterated Benzoins Catalyzed by N-Heterocyclic Carbene [J]. Chinese Journal of Organic Chemistry, 2023, 43(4): 1510-1516. |
| [9] | Siqiang Fang, Zanjiao Liu, Tianli Wang. Recent Advances of the Atherton-Todd Reaction [J]. Chinese Journal of Organic Chemistry, 2023, 43(3): 1069-1083. |
| [10] | Ling Meng, Jun Wang. Research Progress on Synthesis of Thioflavonoids [J]. Chinese Journal of Organic Chemistry, 2023, 43(3): 873-891. |
| [11] | Chunbo Dai, Siqi Xia, Xiaoyu Chen, Limin Yang. N-Heterocyclic Carbene (NHC)-Catalyzed [4+3] Cycloaddition to Synthesize 4-Aminobenzoheptenolactons [J]. Chinese Journal of Organic Chemistry, 2023, 43(3): 1084-1090. |
| [12] | Haiqing Wang, Shuang Yang, Yuchen Zhang, Feng Shi. Advances in Catalytic Asymmetric Reactions Involving o-Hydroxybenzyl Alcohols [J]. Chinese Journal of Organic Chemistry, 2023, 43(3): 974-999. |
| [13] | Weidi Cao, Xiaohua Liu. Recent Advances on Catalytic Enantioselective Protonation for Construction of α-Tertiary Carbonyl Compounds [J]. Chinese Journal of Organic Chemistry, 2023, 43(3): 961-973. |
| [14] | Yushan Zhang, Zhen Huan, Jindong Yang, Jinpei Cheng. Recent Advances in Hydrogen Transfer Reactivities of N-Heterocyclic Phosphines [J]. Chinese Journal of Organic Chemistry, 2023, 43(11): 3806-3825. |
| [15] | Yan Zeng, Fei Ye. Research Progress on New Catalytic Reaction Systems for Asymmetric Synthesis of Silicon-Stereogenic Center Containing Compounds [J]. Chinese Journal of Organic Chemistry, 2023, 43(10): 3388-3413. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||