Chinese Journal of Organic Chemistry ›› 2022, Vol. 42 ›› Issue (3): 863-870.DOI: 10.6023/cjoc202108025 Previous Articles Next Articles
ARTICLES
彭文昶a, 王辉a, 张丹维a,*( ), 黎占亭a,b,*(
), 黎占亭a,b,*( )
)
收稿日期:2021-08-17
修回日期:2021-10-27
发布日期:2021-11-03
通讯作者:
张丹维, 黎占亭
基金资助:
Wen-Chang Penga, Hui Wanga, Dan-Wei Zhanga( ), Zhan-Ting Lia,b(
), Zhan-Ting Lia,b( )
)
Received:2021-08-17
Revised:2021-10-27
Published:2021-11-03
Contact:
Dan-Wei Zhang, Zhan-Ting Li
Supported by:Share
Wen-Chang Peng, Hui Wang, Dan-Wei Zhang, Zhan-Ting Li. Folding and Aggregation of Oligoviologens in Water and Cucurbit[n]uril (n=7, 8) Modulation[J]. Chinese Journal of Organic Chemistry, 2022, 42(3): 863-870.
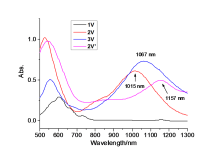

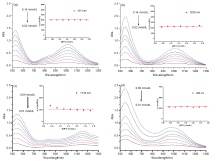



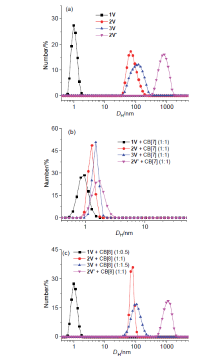
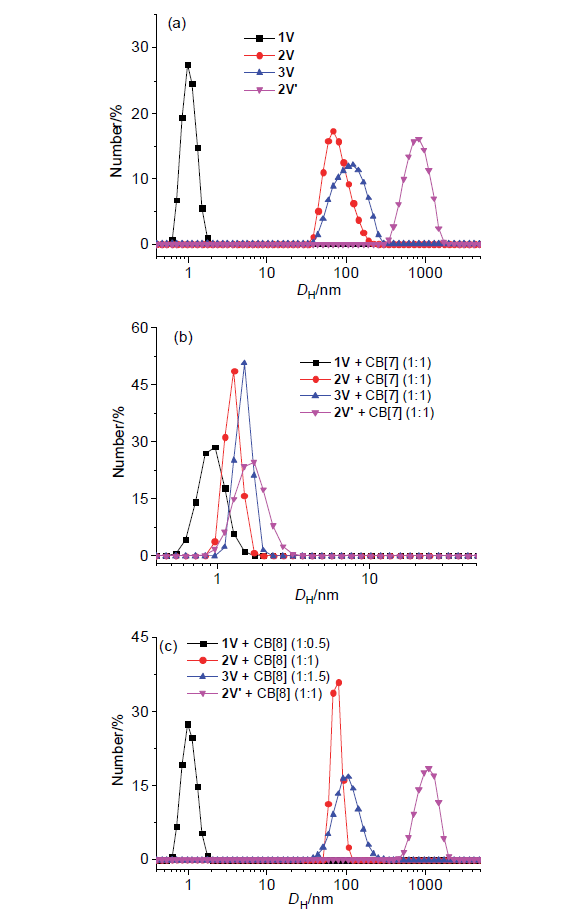
| [1] |
Creighton, T. E. Proteins: Structures and Molecular Principles, 2nd ed., Freeman, New York, 1993.
|
| [2] |
Saenger, W. Principles of Nucleic Acid Structure, Springer-Verlag, New York, 1984.
|
| [3] |
(a) Gellman, S. H. Acc. Chem. Res. 1998, 31, 173.
doi: 10.1021/ar960298r pmid: 20449051 |
|
(b) Hill, D. J.; Mio, M. J.; Prince, R. B.; Hughes, T. S.; Moore, J. S. Chem. Rev. 2001, 101, 3893.
pmid: 20449051 |
|
|
(c) Huc, I. Eur. J. Org. Chem. 2004, 1, 17.
pmid: 20449051 |
|
|
(d) Gong, B. Acc. Chem. Res. 2008, 41, 1376.
doi: 10.1021/ar700266f pmid: 20449051 |
|
|
(e) Li, X.; Wu, Y.-D.; Yang, D. Acc. Chem. Res. 2008, 41, 1428.
doi: 10.1021/ar8001393 pmid: 20449051 |
|
|
(f) Juwarker, H.; Suk, J.; Jeong, K.-S. Chem. Soc. Rev. 2009, 38, 3316.
doi: 10.1039/b909034g pmid: 20449051 |
|
|
(g) Gan, Q.; Wang, Y.; Jiang, H. Curr. Org. Chem. 2011, 15, 1293.
doi: 10.2174/138527211795378227 pmid: 20449051 |
|
|
(h) Zhao, Y.; Cho, H.; Widanapathirana, L.; Zhang, S. Acc. Chem. Res. 2013, 46, 2763.
doi: 10.1021/ar300337f pmid: 20449051 |
|
|
(i) Nair, R. V.; Vijayadas, K. N.; Roy, A.; Sanjayan, G. J. Eur. J. Org. Chem. 2014, 35, 7763.
pmid: 20449051 |
|
|
(j) Huo, Y.-P.; Zeng, H.-Q. Acc. Chem. Res. 2016, 49, 922.
doi: 10.1021/acs.accounts.6b00051 pmid: 20449051 |
|
|
(k) Shigeno, M.; Kushida, Y.; Yamaguchi, M. Chem. Commun. 2016, 52, 4955.
doi: 10.1039/C5CC10379G pmid: 20449051 |
|
|
(l) Zheng, D.; Yu, C.-Y.; Zheng, L.; Zhan, Y.-L.; Jiang, H. Chin. Chem. Lett. 2020, 31, 673.
doi: 10.1016/j.cclet.2019.07.061 pmid: 20449051 |
|
| [4] |
(a) Juwarker, H.; Jeong, K.-S. Chem. Soc. Rev. 2010, 39, 3664.
doi: 10.1039/b926162c pmid: 20730154 |
|
(b) Ferrand, Y.; Huc, I. Acc. Chem. Res. 2018, 51, 970.
doi: 10.1021/acs.accounts.8b00075 pmid: 20730154 |
|
|
(c) Zhao, Y. Curr. Opin. Colloid Interface Sci. 2007, 12, 92.
doi: 10.1016/j.cocis.2007.05.001 pmid: 20730154 |
|
| [5] |
(a) Xin, P.-Y.; Zhu, P.-P.; Su, P.; Hou, J.-L.; Li, Z.-T. J. Am. Chem. Soc. 2014, 136, 1307.
|
|
(b) Zhang, D.-W.; Wang, H.; Li, Z.-T. Prog. Chem. 2020, 32, 1665.
|
|
|
(c) Lang, C.; Li, W.-F.; Dong, Z.-Y.; Zhang, X.; Yang, F.-H.; Yang, B.; Deng, X.-L.; Zhang, C.-Y.; Xu, J.-Y.; Liu, J.-Q. Angew. Chem., Int. Ed. 2016, 55, 9723.
doi: 10.1002/anie.201604071 |
|
|
(d) Chen, F.; Shen, J.; Li, N.; Roy, A.; Ye, R.-J.; Ren, C.-L.; Zeng, H.-Q. Angew. Chem., Int. Ed. 2020, 59, 1440.
doi: 10.1002/anie.v59.4 |
|
| [6] |
(a) Khan, A.; Kaiser, C.; Hecht, S. Angew. Chem., Int. Ed. 2006, 45, 12, 1878.
pmid: 30465607 |
|
(b) Zhang, K.-D.; Zhao, X.; Wang, G.-T.; Liu, Y.; Zhang, Y.; Lu, H.-J.; Jiang, X.-K.; Li, Z.-T. Angew. Chem., nt. Ed. 2011, 50, 9866.
pmid: 30465607 |
|
|
(c) Chen, L.; Wang, H.; Zhang, D.-W.; Zhou, Y.-M.; Li, Z.-T. Angew. Chem., Int. Ed. 2015, 54, 4028.
doi: 10.1002/anie.201410757 pmid: 30465607 |
|
|
(d) Wang, Y.; Bie, F.-S.; Jiang, H. Org. Lett. 2010, 12, 3630.
doi: 10.1021/ol1014043 pmid: 30465607 |
|
|
(e) Parks, F. C.; Liu, Y.; Debnath, S.; Stutsman, S. R.; Raghavachari, K.; Flood, A. H. J. Am. Chem. Soc. 2018, 140, 17711.
doi: 10.1021/jacs.8b10538 pmid: 30465607 |
|
|
(f) Pramanik, S.; Kauffmann, B.; Hecht, S.; Ferrand, Y.; Huc, I. Chem. Commun. 2021, 57, 93.
doi: 10.1039/D0CC06484J pmid: 30465607 |
|
| [7] |
(a) Stone, M. T.; Moore, J. S. Org. Lett. 2004, 6, 469.
doi: 10.1021/ol036238k pmid: 30730716 |
|
(b) Sinkeldam, R. W.; van Houtem, M. H. C. J.; Pieterse, K.; Vekemans, J. A. J. M.; Meijer, E. W. Chem.-Eur. J. 2006, 12, 6129.
pmid: 30730716 |
|
|
(c) Gillies, E. R.; Deiss, F.; Staedel, C.; Schmitter, J.-M.; Huc, I. Angew. Chem., Int. Ed. 2007, 46, 4081.
doi: 10.1002/(ISSN)1521-3773 pmid: 30730716 |
|
|
(d) Wang, Y.; Li, F.; Han, Y.-M.; Wang, F.-Y.; Jiang, H. Chem. Eur. J. 2009, 15, 9424.
doi: 10.1002/chem.v15:37 pmid: 30730716 |
|
|
(e) Suk, J.-M.; Jeong, K.-S. J. Am. Chem. Soc. 2008, 130, 11868.
doi: 10.1021/ja804845m pmid: 30730716 |
|
|
(f) Zhang, P.; Wang, Z.-K.; Zhang, L.; Wang, H.; Zhang, D.-W.; Hou, J.-L.; Li, Z.-T. Chin. J. Chem. 2016, 34, 678.
doi: 10.1002/cjoc.v34.7 pmid: 30730716 |
|
|
(g) Shang, J.; Zhao, W.; Li, X.-C.; Wang, Y.; Jiang, H. Chem. Commun. 2016, 52, 4505.
doi: 10.1039/C5CC10422J pmid: 30730716 |
|
|
(h) Ikkanda, B. A.; Iverson, B. L. Chem. Commun. 2016, 52, 7752.
doi: 10.1039/C6CC01861K pmid: 30730716 |
|
|
(i) Borissov, A.; Marques, I.; Lim, J. Y. C.; Felix, V.; Smith, Ma. D.; Beer, P. D. J. Am. Chem. Soc. 2019, 141, 4119.
doi: 10.1021/jacs.9b00148 pmid: 30730716 |
|
| [8] |
(a) Chen, L.; Wang, H.; Zhang, D.-W.; Zhou, Y.-M.; Li, Z.-T. Angew. Chem., Int. Ed. 2015, 54, 4028.
doi: 10.1002/anie.201410757 |
|
(b) Zhang, Y.-C.; Zhang, D.-W.; Wang, H.; Zhou, Y.-M.; Li, Z.-T. Polym. Chem. 2015, 6, 4404.
doi: 10.1039/C5PY00419E |
|
|
(c) Chen, L.; Wang, H.; Zhang, D.-W.; Zhou, Y.-M.; Li, Z.-T. Tetrahedron 2017, 73, 1841.
doi: 10.1016/j.tet.2017.02.034 |
|
|
(d) Qi, Q.; Yang, B.; Xi, C.-G.; Yang, X.-R.; Zhang, D.-W.; Liu, S.-M.; Li, Z.-T. ChemistrySelect 2016, 1, 6792.
doi: 10.1002/slct.201601760 |
|
| [9] |
Wang, Y.-P.; Frasconi, M.; Liu, W.-G.; Liu, Z.-C.; Sarjeant, A. A.; Nassar, M. S.; Botros, Y. Y.; Goddard, W. A.; Stoddart, J. F. J. Am. Chem. Soc. 2015, 137, 876.
doi: 10.1021/ja5111305 |
| [10] |
(a) Zhang, D.-W.; Tian, J.; Chen, L.; Zhang, L.; Li, Z.-T. Chem. Asian J. 2015, 10, 56.
doi: 10.1002/asia.201402805 |
|
(b) Chen, L.; Zhang, Y.-C.; Wang, W.-K.; Tian, J.; Zhang, L.; Wang, H.; Zhang, D.-W.; Li, Z.-T. Chin. Chem. Lett. 2015, 26, 811.
doi: 10.1016/j.cclet.2015.01.036 |
|
|
(c) Zhou, X.-H.; Fan, Y.; Li, W.-X.; Zhang, X.; Liang, R.-R.; Lin, F.; Zhan, T.-G.; Jiecheng Cui, J.; Liu, L.-J.; Zhao, X. Zhang, K.-D. Chin. Chem. Lett. 2020, 31, 1757.
doi: 10.1016/j.cclet.2019.12.039 |
|
| [11] |
Wang, Y.-P.; Frasconi, M.; Liu, W.-G.; Sun, J.-L.; Wu, Y.-L.; Nassar, M. S.; Botros, Y. Y.; Goddard, W. A.; Wasielewski, M. R.; Stoddart, J. F. ACS Cent. Sci. 2016, 2, 89.
doi: 10.1021/acscentsci.5b00377 |
| [12] |
Zhou, C.; Tian, J.; Wang, J.-L.; Zhang, D.-W.; Zhao, X.; Liu, Y.; Li, Z.-T. Polym. Chem. 2014, 5, 341.
doi: 10.1039/C3PY01006F |
| [13] |
Liu, H. Supramol. Chem. 2019, 31, 451.
doi: 10.1080/10610278.2019.1622702 |
| [14] |
(a) Liu, X.-B.; Lin, J.-L.; Wang, H.; Zhang, D.-W.; Li, Z.-T. Chin. J. Org. Chem. 2020, 40, 663 (in Chinese)
|
|
(刘旭波, 林佳乐, 王辉, 张丹维, 黎占亭, 有机化学, 2020, 40, 663.)
doi: 10.6023/cjoc201910022 |
|
|
(b) Tian, X.; Zuo, M.; Niu, P.; Wang, K.; Hu, X. Chin. J. Org. Chem. 2020, 40, 1823. (in Chinese)
doi: 10.6023/cjoc202003066 |
|
|
(田雪琪, 左旻瓒, 牛蓬勃, 王开亚, 胡晓玉, 有机化学, 2020, 40, 1823.)
doi: 10.6023/cjoc202003066 |
|
| [15] |
(a) Correia, H. D.; Chowdhury, S.; Ramos, A. P.; Guy, L.; Demets, G. J.-F.; Bucher, C. Polym. Int. 2019, 68, 572.
doi: 10.1002/pi.2019.68.issue-4 |
|
(b) Zhou, J.; Hou, S.; Zhang, J.; Chen, Y.; Chen, H.; Tan, Y. Chin. Chem. Lett. 2021, 32, 725.
doi: 10.1016/j.cclet.2020.07.039 |
|
|
(c) Ong, W.; Gomez-Kaifer, M.; Kaifer, A. E. Org. Lett. 2002, 4, 1791.
doi: 10.1021/ol025869w |
|
| [16] |
Yang, B.; Zhang, J.-W.; Yu, S.-B.; Wang, Z.-K.; Zhang, P.-Q.; Yang, X.-D.; Qi, Q.-Y.; Yang, G.-Y.; Ma, D.; Li, Z.-T. Sci. China Chem. 2021, 64, 1228.
doi: 10.1007/s11426-021-1012-5 |
| [17] |
(a) Yin, Z.; Song, G.; Jiao, Y.; Zheng, P.; Xu, J.-F.; Zhang, X. CCS Chem. 2019, 1, 335.
doi: 10.31635/ccschem.019.20190013 |
|
(b) Ji, Q.; Fan, L.; Liu, S.; Ye, H.; Xiang, S.; Wang, P. Chin. Chem. Lett. 2021, 10.1016/j.cclet.2021.05.036.
|
|
| [18] |
Geuder, W.; Hunig, S.; Suchy, A. Tetrahedron 1986, 42, 1665.
doi: 10.1016/S0040-4020(01)87583-9 |
| [19] |
Yang, B.; Yu, S.-B.; Wang, H.; Zhang, D.-W.; Li, Z.-T. Chem. Asian J. 2018, 13, 1312.
doi: 10.1002/asia.v13.10 |
| [1] | Chongyang Zeng, Ping Hu, Biqin Wang, Wenyan Fang, Keqing Zhao. Cyanostilbene Bridged Triphenylene Dyad Stimuli-Responsive Discotic Liquid Crystal: Synthesis, Properties and Applications [J]. Chinese Journal of Organic Chemistry, 2023, 43(9): 3287-3296. |
| [2] | Yang Zhao, Panpan Chen, Lizhi Han, Enju Wang. Aggregation-Induced Emission and Cell Imaging of Triphenylimidazole Derivatives [J]. Chinese Journal of Organic Chemistry, 2023, 43(7): 2454-2461. |
| [3] | Ling Liu, Taotao Hao, Wanhua Wu, Cheng Yang. Stilbene-Based Molecular Switches with Aggregation Induced Emission (AIE) Function Constructed by Supramolecular Strategy [J]. Chinese Journal of Organic Chemistry, 2023, 43(6): 2189-2196. |
| [4] | Yang Zhao, Panpan Chen, Gaonan Li, Zhigang Niu, Enju Wang. Tetraarylimidazole-Based Aggregation-Induced Emission Luminogens and Their Cell-Imaging Application [J]. Chinese Journal of Organic Chemistry, 2023, 43(6): 2156-2162. |
| [5] | Yuehua Zhang, Fei Nie, Lu Zhou, Xiaofeng Wang, Yuan Liu, Yanping Huo, Wencheng Chen, Zujin Zhao. Synthesis and Optoelectronic Studies of Thermally Activated Delayed Fluorescence Materials Based on Benzothiazolyl Ketones [J]. Chinese Journal of Organic Chemistry, 2023, 43(11): 3876-3887. |
| [6] | Meng Liu, Yanru Huang, Xiaofei Sun, Lijun Tang. An “Aggregation-Induced Emission+Excited-State Intramolecular Proton Transfer” Mechanisms-Based Benzothiazole Derived Fluorescent Probe and Its ClO– Recognition [J]. Chinese Journal of Organic Chemistry, 2023, 43(1): 345-351. |
| [7] | Yangyang Li, Xiaofei Sun, Xiaoling Hu, Yuanyuan Ren, Keli Zhong, Xiaomei Yan, Lijun Tang. Synthesis of Triphenylamine Derivative and Its Recognition for Hg2+ with “OFF-ON” Fluorescence Response Based on Aggregation-Induced Emission (AIE) Mechanism [J]. Chinese Journal of Organic Chemistry, 2023, 43(1): 320-325. |
| [8] | Jidong Zhang, Wanlin Yan, Wenqiang Hu, Dian Guo, Dalong Zhang, Xiaoxin Quan, Xianpan Bu, Siyu Chen. Design and Synthesis of a Zn2+ Fluorescent Probe Based on Aggregation Induced Luminescence Properties [J]. Chinese Journal of Organic Chemistry, 2023, 43(1): 326-331. |
| [9] | Weikang Xia, Chuang Liu, Sheng Ye, Lei Wang, Ruiyuan Liu. Synthesis of A Sulfonamide-Substituted Benzothiadiazole-Based Fluorescent Dye and Study of Its Application for Long-Term Cancer Cell Tracking [J]. Chinese Journal of Organic Chemistry, 2022, 42(8): 2535-2541. |
| [10] | Zhaohua Chen, Xiying Cao, Sihong Chen, Shiwei Yu, Yanlan Lin, Shuting Lin, Zhaoyang Wang. Design, Synthesis and Application of Trisubstituted Olefinic Aggregation-Induced Emission Molecules [J]. Chinese Journal of Organic Chemistry, 2022, 42(8): 2355-2363. |
| [11] | Ze Guo, Di Wu, Lili Wang, Zheng Duan. BF3•Et2O Promoted Dienone-Phenol Type Rearrangement to Synthesize Phosphepine with Aggregation Induced Luminescence (AIE) Effect [J]. Chinese Journal of Organic Chemistry, 2022, 42(8): 2481-2487. |
| [12] | Yuetian Guo, Yongxin Pan, Lijun Tang. Progresses in Reactive Fluorescent Probes with Fused Aggregation- Induced Emission (AIE) and Excited State Intramolecular Proton Transfer (ESIPT) Structures [J]. Chinese Journal of Organic Chemistry, 2022, 42(6): 1640-1650. |
| [13] | Sihong Chen, Jiamin Xu, Yuemei Li, Baoru Peng, Lingyu Luo, Huiye Feng, Zhaohua Chen, Zhaoyang Wang. Research Progress of Aggregation-Caused Quenching (ACQ) to Aggregation-Induced Emission (AIE) Transformation Based on Organic Small Molecules [J]. Chinese Journal of Organic Chemistry, 2022, 42(6): 1651-1666. |
| [14] | Yiping Wen, Zhengfeng Xie, Tianzhu Shi, Yicheng Chu, Ronggui Zhou, Yishan Tao, Huanmin Liang, Haiyan Qiu, Yunhui Zhao. Synthesis of Triazole Functionalized Triphenylamine Cu2+ Fluorescent Probe and Its Application in Detection and HeLa Cells [J]. Chinese Journal of Organic Chemistry, 2022, 42(5): 1463-1473. |
| [15] | Yongmei Zhao, Yeshu Mu, Wen Luo, Zhiyong Tian. Synthesis of Naphthalimide Derivatives as Cholinesterase Inhibitors with Aggregation Induced Emission Properties [J]. Chinese Journal of Organic Chemistry, 2022, 42(3): 819-829. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||