Chinese Journal of Organic Chemistry ›› 2023, Vol. 43 ›› Issue (6): 2189-2196.DOI: 10.6023/cjoc202210020 Previous Articles Next Articles
收稿日期:2022-10-19
修回日期:2022-11-19
发布日期:2022-12-21
基金资助:
Ling Liu, Taotao Hao*( ), Wanhua Wu, Cheng Yang*(
), Wanhua Wu, Cheng Yang*( )
)
Received:2022-10-19
Revised:2022-11-19
Published:2022-12-21
Contact:
E-mail: Supported by:Share
Ling Liu, Taotao Hao, Wanhua Wu, Cheng Yang. Stilbene-Based Molecular Switches with Aggregation Induced Emission (AIE) Function Constructed by Supramolecular Strategy[J]. Chinese Journal of Organic Chemistry, 2023, 43(6): 2189-2196.
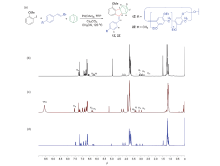
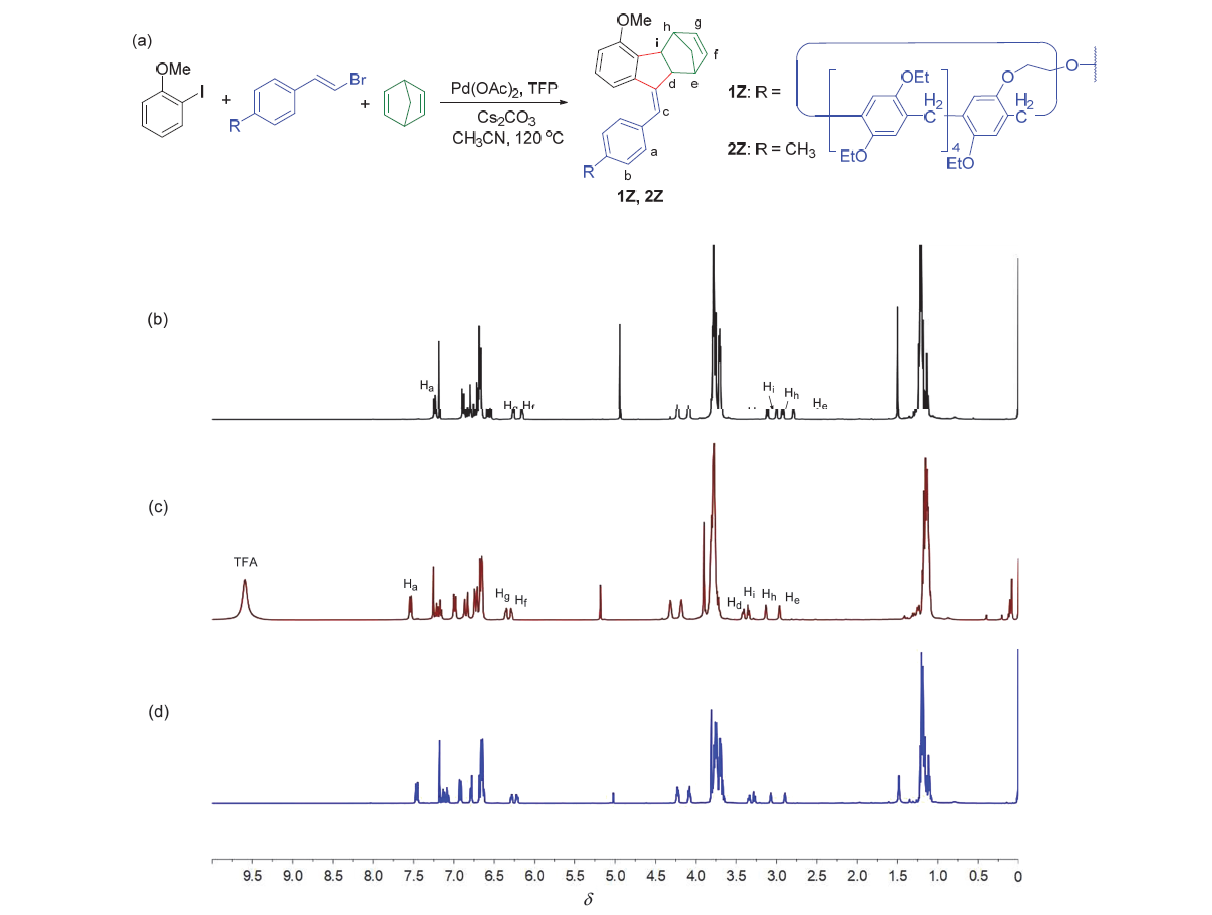


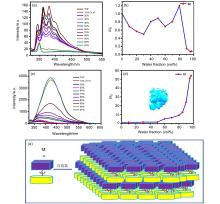



| [1] |
Erbas-Cakmak, S.; Leigh, D. A.; McTernan, C. T.; Nuss-baumer, A. L. Chem. Rev. 2015, 115, 10081.
doi: 10.1021/acs.chemrev.5b00146 pmid: 26346838 |
| [2] |
Irie, M.; Fukaminato, T.; Matsuda, K.; Kobatake, S. Chem. Rev. 2014, 114, 12174.
doi: 10.1021/cr500249p |
| [3] |
Villarón, D.; Wezenberg, S. J. Angew. Chem., Int. Ed. 2020, 59, 2.
|
| [4] |
Koumura, N.; Zijlstra, R. W. J.; Van Delden, R. A.; Harada, N.; Feringa, B. L. Nature 1999, 401, 152.
doi: 10.1038/43646 |
| [5] |
Feringa, B. L. J. Org. Chem. 2007, 72, 6635.
pmid: 17629332 |
| [6] |
Feringa, B. L. Angew. Chem., Int. Ed. 2017, 56, 11060.
doi: 10.1002/anie.201702979 |
| [7] |
Serreli, V.; Lee, C. F.; Kay, E. R.; Leigh, D. A. Nature 2007, 445, 523.
doi: 10.1038/nature05452 |
| [8] |
Waldeck, D. H. Chem. Rev. 1991, 91, 415.
doi: 10.1021/cr00003a007 |
| [9] |
Noyce, D. S.; Hartter, D. R.; Miles, F. B. J. Am. Chem. Soc. 1968, 90, 4633.
doi: 10.1021/ja01019a021 |
| [10] |
(a) Zhang, Q.-Q.; Lin, P.-P.; Yang, L.; Tan, D.-H.; Feng, S.-X.; Wang, H.; Li, Q. Chin. J. Org. Chem. 2020, 40, 3314. (in Chinese)
doi: 10.6023/cjoc202005048 |
|
(张其奇, 林鹏鹏, 杨羚, 谭东航, 冯嗣欣, 王洪根, 李清江, 有机化学, 2020, 40, 3314.)
doi: 10.6023/cjoc202005048 |
|
|
(b) Chen, H.-C.; Wu, Yi.; Yu, Y.; Wang, P. Chin. J. Org. Chem. 2022, 42, 742. (in Chinese)
doi: 10.6023/cjoc202109045 |
|
|
(陈宏超, 吴奕晨, 于洋, 王鹏, 有机化学, 2022, 42, 742.)
doi: 10.6023/cjoc202109045 |
|
| [11] |
Lerch, M. M.; Hansen, M. J.; Velema, W. A.; Szymanski, W.; Feringa, B. L. Nat. Commun. 2016, 7, 12054.
doi: 10.1038/ncomms12054 |
| [12] |
(a) Wang, L.; Li, Q. Chem. Soc. Rev., 2018, 47, 1044.
doi: 10.1039/C7CS00630F |
|
(b) Weng, G.-H.; Zhu, B.; Ye, Y.; Li, S. Chin. J. Org. Chem. 2015, 35, 309. (in Chinese)
doi: 10.6023/cjoc201409021 |
|
|
(翁官欢, 朱彬, 叶杨, 李世军, 有机化学, 2015, 35, 309.)
doi: 10.6023/cjoc201409021 |
|
| [13] |
(a) Luo, J. D.; Xie, Z. L.; Lam, J. W. Y.; Cheng, L.; Chen, H. Y.; Qiu, C. F.; Kwok, H. S.; Zhan, X. W.; Liu, Y. Q.; Zhu, D. B.; Tang, B. Z. Chem. Commun. 2001, 18, 1740.
pmid: 26492387 |
|
(b) Mei, J.; Leung, N. L. C.; Kwok, R. T. K.; Lam, J. W. Y.; Tang, B. Z. Chem. Rev. 2015, 115, 11718.
doi: 10.1021/acs.chemrev.5b00263 pmid: 26492387 |
|
|
(c) Yang, H.; Li, H.; Yue, L.; Chen, X.; Song, D.; Yang, X.; Sun, Y.; Zhou, G.; Wu, Z. J. Mater. Chem. C 2021, 9, 2334.
doi: 10.1039/D0TC05527A pmid: 26492387 |
|
|
(d) Zhao, J.; Feng, Z.; Zhong, D.; Yang, X.; Wu, Y.; Zhou, G.; Wu, Z. Chem. Mater. 2018, 30, 929.
doi: 10.1021/acs.chemmater.7b04708 pmid: 26492387 |
|
|
(e) Zhao, J.; Dang, F.; Feng, Z.; Liu, B.; Yang, X.; Wu, Y.; Zhou, G.; Wu, Z.; Wong, W.-Y. Chem. Commun., 2017, 53, 7581.
doi: 10.1039/C7CC03251J pmid: 26492387 |
|
| [14] |
Wei, P.; Zhang, J.-X.; Zhao, Z.; Chen, Y.; He, X.; Chen, M.; Gong, J.; Sung, H.-H.; Williams, I. D.; Lam, J. W. Y.; Tang, B.-Z. J. Am. Chem. Soc. 2018, 140, 1966.
doi: 10.1021/jacs.7b13364 |
| [15] |
(a) Yao, J.; Wu, W.; Liang, W.; Feng, Y.; Zhou, D.; Chruma, J. J.; Fukuhara, G.; Mori, T.; Inoue, Y.; Yang, C. Angew. Chem., Int. Ed. 2017, 56, 6869.
doi: 10.1002/anie.201702542 |
|
(b) Xiao, C.; Wu, W.; Liang, W.; Zhou, D.; Kanagaraj, K.; Cheng, G.; Su, D.; Zhong, Z.; Chruma, J. J.; Yang, C. Angew. Chem., Int. Ed. 2020, 132, 8171.
doi: 10.1002/ange.v132.21 |
|
|
(c) Yao, J.; Wu, W.; Xiao, C.; Su, D.; Zhong, Z.; Mori, T.; Yang, C. Nat. Commun. 2021, 12, 2600.
doi: 10.1038/s41467-021-22880-z |
|
|
(d) Peng, C.; Liang, W.; Ji, J.; Fan, C.; Kanagaraj, K.; Wu, W.; Cheng, G.; Su, D.; Zhong, Z.; Yang, C. Chin. Chem. Lett., 2021, 32, 345.
doi: 10.1016/j.cclet.2020.03.079 |
|
|
(e) Yu, X.; Wu, W.; Zhou, D.; Su, D.; Zhong, Z.; Yang, C. CCS Chemistry 2021, 3, 1945.
|
|
|
(f) Zhang, D.; Liang, W.; Yi, J.; Chen, J.; Lv, Y.; Zhao, T.; Xiao, C.; Xie, X.; Wu, W.; Yang, C. Sci. China Chem. 2022, 65, 1149.
doi: 10.1007/s11426-022-1233-x |
|
| [16] |
Hao, T.-T.; Yang, Y.-S.; Liang, W.-T.; Fan, C.-Y.; Wang, X.; Wu, W.-H.; Chen, X.-C.; Fu, H.-Y.; Chen, H.; Yang, C. Chem. Sci. 2021, 12, 2614.
doi: 10.1039/D0SC05213B |
| [17] |
Hao, T.-T.; Liang, H.-R.; Ou-Yang, Y.-H.; Yin, C.-Z.; Zheng, X.-L.; Yuan, M.-L.; Li, R.-X.; Fu, H.-Y.; Chen, H. J. Org. Chem. 2018, 83, 4441.
doi: 10.1021/acs.joc.8b00150 |
| [18] |
(a) Tian, X.; Zuo, M.; Niu, P.; Wang, K.; Hu, X. Chin. J. Org. Chem. 2020, 40, 1823. (in Chinese)
doi: 10.6023/cjoc202003066 |
|
(田雪琪, 左旻瓒, 牛蓬勃, 王开亚, 胡晓玉, 有机化学, 2020, 40, 1823.)
doi: 10.6023/cjoc202003066 |
|
|
(b) Bian, S.; Ye, J.-H.; Fan, Z.; Zhang, W. C.; Wang, L. Y. Chin. J. Org. Chem. 2016, 36, 855. (in Chinese)
doi: 10.6023/cjoc201510030 |
|
|
(卞松, 叶家海, 樊政, 张文超, 王乐勇, 有机化学, 2016, 36, 855.)
doi: 10.6023/cjoc201510030 |
|
| [19] |
(a) Zhang, H.; Liu, Z.; Xin, F.; Hao, A. Chin. J. Org. Chem. 2012, 32, 219. (in Chinese)
|
|
(张华承, 刘召娜, 辛飞飞, 郝爱友, 有机化学, 2012, 32, 219.)
doi: 10.6023/cjoc1107141 |
|
|
(b) Wu, X.; Duan, Q.; Ni, M.; Hu, X.; Wang, L. Chin. J. Org. Chem. 2014, 34, 437. (in Chinese)
|
|
|
(吴旋, 段群鹏, 倪梦飞, 胡晓玉, 王乐勇, 有机化学, 2014, 34, 437.)
doi: 10.6023/cjoc201312018 |
|
|
(c) Wang, Z.; Liu, Y. A.; Yang, H.; Hu, W.; Wen, K. Org. Lett. 2022, 24, 1822.
doi: 10.1021/acs.orglett.2c00272 |
|
| [20] |
Petermayer, C.; Dube, H. Acc. Chem. Res. 2018, 51, 1153.
doi: 10.1021/acs.accounts.7b00638 |
| [21] |
Goulet-Hanssens, A.; Utecht, M.; Mutruc, D.; Titov, E.; Schwarz, J.; Grubert, L.; Bleger, D.; Saalfrank, P.; Hecht, S. J. Am. Chem. Soc. 2017, 139, 335.
doi: 10.1021/jacs.6b10822 pmid: 27997152 |
| [22] |
Fredrich, S.; Bonasera, A.; Valderrey, V.; Hecht, S. J. Am. Chem. Soc. 2018, 140, 6432.
doi: 10.1021/jacs.8b02982 |
| [23] |
Konrad, D. B.; Savasci, G.; Allmendinger, L.; Trauner, D.; Ochsenfeld, C.; Ali, A. M. J. Am. Chem. Soc. 2020, 142, 6538.
doi: 10.1021/jacs.9b10430 pmid: 32207943 |
| [24] |
Hnid, I.; Frath, D.; Lafolet, F.; Sun, X.; Lacroix, J. C. J. Am. Chem. Soc. 2020, 142, 7732.
doi: 10.1021/jacs.0c01213 |
| [25] |
Yin, J.; Khalilov, A. N.; Muthupandi, P.; Ladd, R.; Birman, V. B. J. Am. Chem. Soc. 2020, 142, 60.
doi: 10.1021/jacs.9b11160 |
| [26] |
Luo, Q.; Fan, Q.; Huang, W. Chin. J. Org. Chem. 2007, 27, 175. (in Chinese)
|
|
(罗千福, 范曲立, 黄维, 有机化学, 2007, 27, 175.)
|
|
| [27] |
Feringa, B. L.; van Delden, R. A.; Koumura, N.; Geertsema, E. M. Chem. Rev. 2000, 100, 1789.
pmid: 11777421 |
| [28] |
Wang, Y.; Li, Q. Adv. Mater. 2012, 24, 1926.
doi: 10.1002/adma.201200241 |
| [29] |
Fa, S.; Mizobata, M.; Nagano, S.; Suetsugu, K.; Kakuta, T.; Yamagishi, T.; Ogoshi, T. ACS Nano 2021, 15, 16794.
doi: 10.1021/acsnano.1c06975 |
| [30] |
Zhao, D.; Neubauer, T. M.; Feringa1, B. L. Nat. Commun. 2015, 6, 6652.
doi: 10.1038/ncomms7652 |
| [31] |
Blanco, V.; Leigh, D. A.; Marcos, V. Chem. Soc. Rev. 2015, 44, 5341.
doi: 10.1039/C5CS00096C |
| [32] |
Ryabchun, A.; Li, Q.; Lancia, F.; Aprahamian, I.; Katsonis, N. J. Am. Chem. Soc. 2019, 141, 1196.
doi: 10.1021/jacs.8b11558 |
| [33] |
Mei, J.; Hong, Y. N.; Lam, J. W. Y.; Qin, A. J.; Tang, Y. H.; Tang, B. Z. Adv. Mater. 2014, 26, 5429.
doi: 10.1002/adma.201401356 |
| [34] |
Xia, D.; Wang, P.; Ji, X.; Khashab, N. M.; Sessler, J. L.; Huang, F. Chem. Rev. 2020, 120, 6070.
doi: 10.1021/acs.chemrev.9b00839 |
| [35] |
Xu, M.; Li, N.; Zhao, Z.; Shi, Z.; S, J.; Chen, L. Eur. J. Med. Chem. 2019, 174, 265.
doi: 10.1016/j.ejmech.2019.04.050 |
| [36] |
Chen, J.; Ni, H.; Meng, Z.; Wang, J.; Huang, X.; Dong, Y.; Sun, C.; Zhang, Y.; Cui, L.; Li, J.; Jia, X.; Meng, Q.; Li, C. Nat. Commun. 2019, 10, 3546.
doi: 10.1038/s41467-019-11553-7 |
| [1] | Chongyang Zeng, Ping Hu, Biqin Wang, Wenyan Fang, Keqing Zhao. Cyanostilbene Bridged Triphenylene Dyad Stimuli-Responsive Discotic Liquid Crystal: Synthesis, Properties and Applications [J]. Chinese Journal of Organic Chemistry, 2023, 43(9): 3287-3296. |
| [2] | Yang Zhao, Panpan Chen, Lizhi Han, Enju Wang. Aggregation-Induced Emission and Cell Imaging of Triphenylimidazole Derivatives [J]. Chinese Journal of Organic Chemistry, 2023, 43(7): 2454-2461. |
| [3] | Yang Zhao, Panpan Chen, Gaonan Li, Zhigang Niu, Enju Wang. Tetraarylimidazole-Based Aggregation-Induced Emission Luminogens and Their Cell-Imaging Application [J]. Chinese Journal of Organic Chemistry, 2023, 43(6): 2156-2162. |
| [4] | Yuehua Zhang, Fei Nie, Lu Zhou, Xiaofeng Wang, Yuan Liu, Yanping Huo, Wencheng Chen, Zujin Zhao. Synthesis and Optoelectronic Studies of Thermally Activated Delayed Fluorescence Materials Based on Benzothiazolyl Ketones [J]. Chinese Journal of Organic Chemistry, 2023, 43(11): 3876-3887. |
| [5] | Meng Liu, Yanru Huang, Xiaofei Sun, Lijun Tang. An “Aggregation-Induced Emission+Excited-State Intramolecular Proton Transfer” Mechanisms-Based Benzothiazole Derived Fluorescent Probe and Its ClO– Recognition [J]. Chinese Journal of Organic Chemistry, 2023, 43(1): 345-351. |
| [6] | Yangyang Li, Xiaofei Sun, Xiaoling Hu, Yuanyuan Ren, Keli Zhong, Xiaomei Yan, Lijun Tang. Synthesis of Triphenylamine Derivative and Its Recognition for Hg2+ with “OFF-ON” Fluorescence Response Based on Aggregation-Induced Emission (AIE) Mechanism [J]. Chinese Journal of Organic Chemistry, 2023, 43(1): 320-325. |
| [7] | Jidong Zhang, Wanlin Yan, Wenqiang Hu, Dian Guo, Dalong Zhang, Xiaoxin Quan, Xianpan Bu, Siyu Chen. Design and Synthesis of a Zn2+ Fluorescent Probe Based on Aggregation Induced Luminescence Properties [J]. Chinese Journal of Organic Chemistry, 2023, 43(1): 326-331. |
| [8] | Weikang Xia, Chuang Liu, Sheng Ye, Lei Wang, Ruiyuan Liu. Synthesis of A Sulfonamide-Substituted Benzothiadiazole-Based Fluorescent Dye and Study of Its Application for Long-Term Cancer Cell Tracking [J]. Chinese Journal of Organic Chemistry, 2022, 42(8): 2535-2541. |
| [9] | Zhaohua Chen, Xiying Cao, Sihong Chen, Shiwei Yu, Yanlan Lin, Shuting Lin, Zhaoyang Wang. Design, Synthesis and Application of Trisubstituted Olefinic Aggregation-Induced Emission Molecules [J]. Chinese Journal of Organic Chemistry, 2022, 42(8): 2355-2363. |
| [10] | Ze Guo, Di Wu, Lili Wang, Zheng Duan. BF3•Et2O Promoted Dienone-Phenol Type Rearrangement to Synthesize Phosphepine with Aggregation Induced Luminescence (AIE) Effect [J]. Chinese Journal of Organic Chemistry, 2022, 42(8): 2481-2487. |
| [11] | Yuetian Guo, Yongxin Pan, Lijun Tang. Progresses in Reactive Fluorescent Probes with Fused Aggregation- Induced Emission (AIE) and Excited State Intramolecular Proton Transfer (ESIPT) Structures [J]. Chinese Journal of Organic Chemistry, 2022, 42(6): 1640-1650. |
| [12] | Sihong Chen, Jiamin Xu, Yuemei Li, Baoru Peng, Lingyu Luo, Huiye Feng, Zhaohua Chen, Zhaoyang Wang. Research Progress of Aggregation-Caused Quenching (ACQ) to Aggregation-Induced Emission (AIE) Transformation Based on Organic Small Molecules [J]. Chinese Journal of Organic Chemistry, 2022, 42(6): 1651-1666. |
| [13] | Wei Ding, Bowen Cheng, Meng Wang, Qingyu Dou, Siying Li, Peng Zhang, Qianfu Luo. Advances in Aggregation-Induced Emission Molecules Based on Organic Photochromism [J]. Chinese Journal of Organic Chemistry, 2022, 42(2): 363-383. |
| [14] | Jiamin Tan, Yajun Yu, Meng Guan, Yunhui Zhao, Zilong Tang, Zhihua Zhou, Tao Guo. Design and Synthesis of Novel Aggregation-Induced Luminescence Molecules Based on Isoquinoline [J]. Chinese Journal of Organic Chemistry, 2022, 42(11): 3776-3783. |
| [15] | Qi Lu, Feixia Ye, Xiaotong Sun, Jianquan Weng, Qian Yu, Dexuan Hu. Design and Synthesis of Novel Nature-Inspired Stilbene Analogues as Potential Topoisomerase 1 Inhibitors [J]. Chinese Journal of Organic Chemistry, 2021, 41(8): 3321-3329. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||