Chinese Journal of Organic Chemistry ›› 2022, Vol. 42 ›› Issue (4): 945-964.DOI: 10.6023/cjoc202110008 Previous Articles Next Articles
REVIEWS
张志豪a,b,c, 姜芯a,b,c, 李清寒a,b,c,*( )
)
收稿日期:2021-10-08
修回日期:2021-11-20
发布日期:2021-12-15
通讯作者:
李清寒
基金资助:
Zhihao Zhanga,b,c, Xin Jianga,b,c, Qinghan Lia,b,c( )
)
Received:2021-10-08
Revised:2021-11-20
Published:2021-12-15
Contact:
Qinghan Li
Supported by:Share
Zhihao Zhang, Xin Jiang, Qinghan Li. Recent Progress in the Synthesis of Substituted Benzo[b]furan Derivatives[J]. Chinese Journal of Organic Chemistry, 2022, 42(4): 945-964.


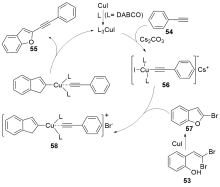



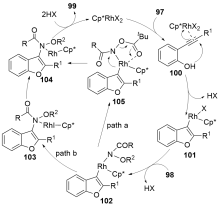
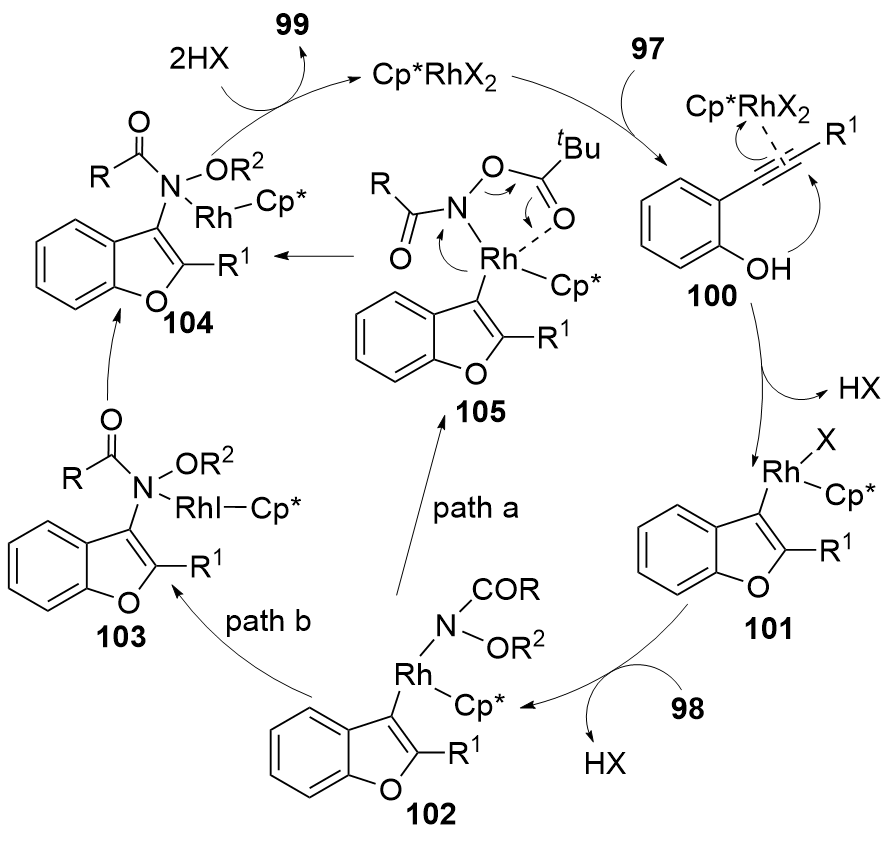
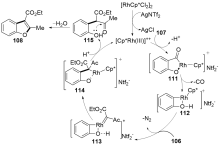


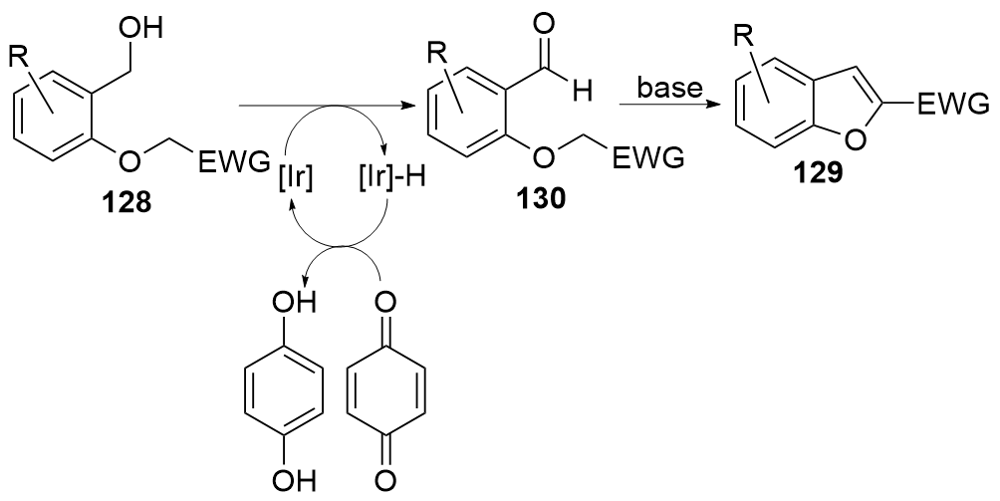

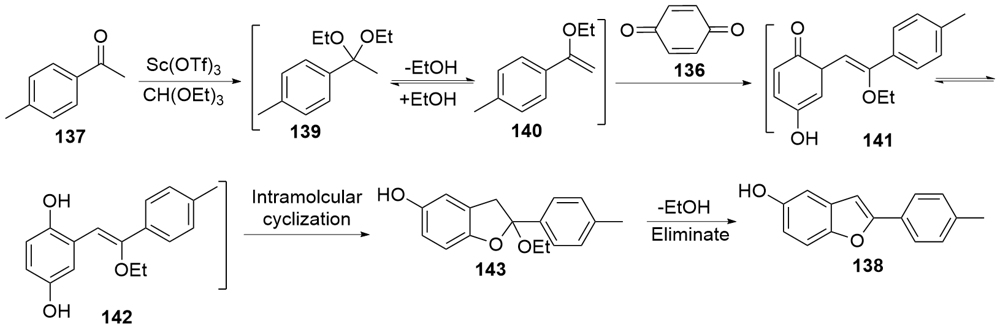

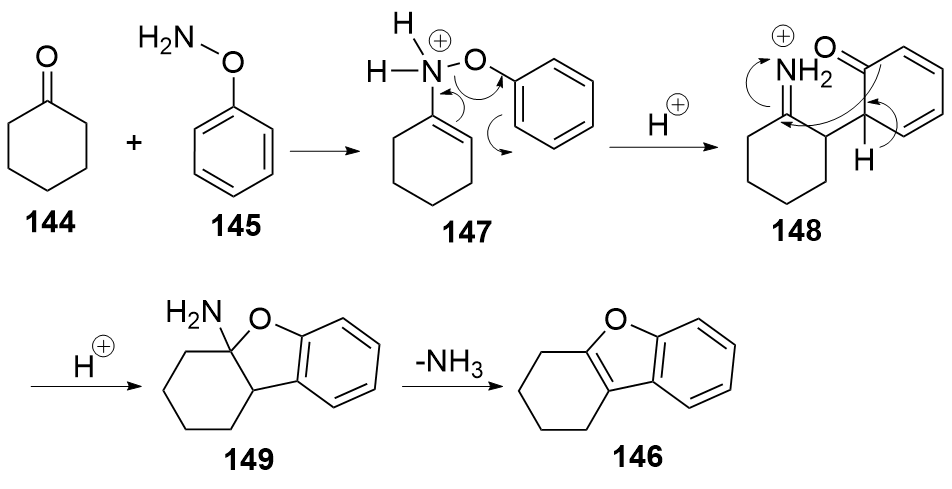
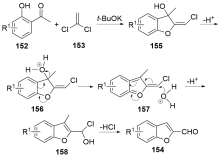


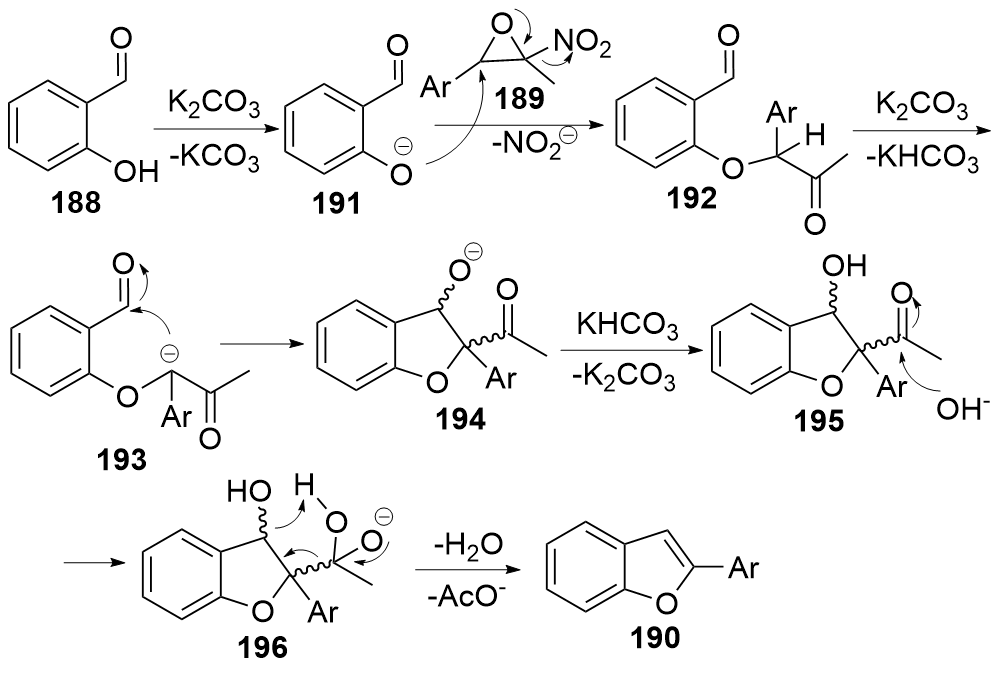
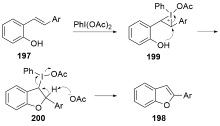
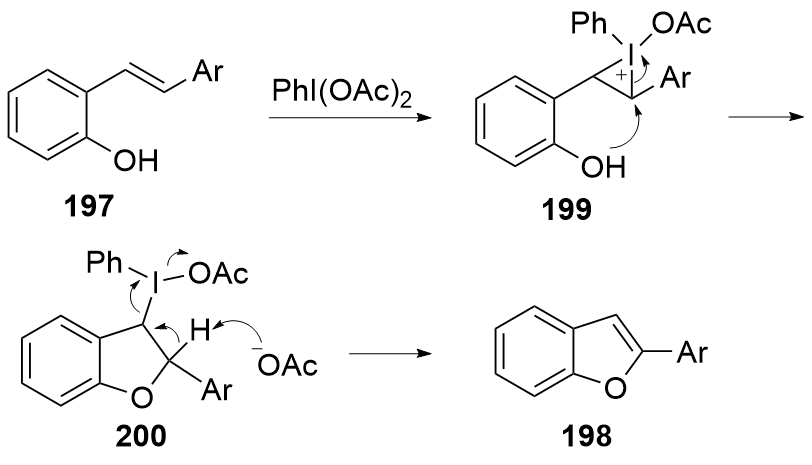
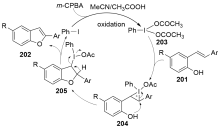
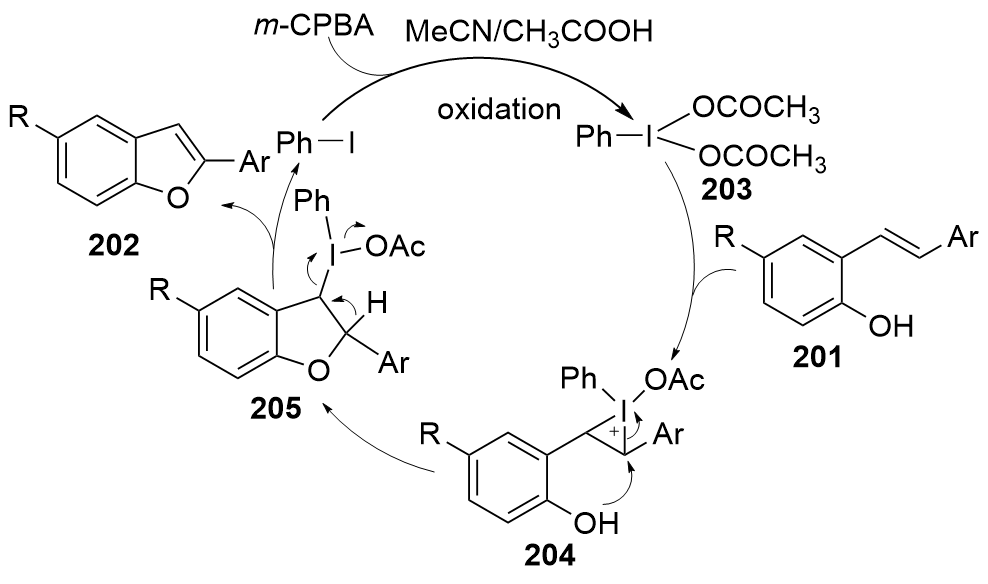
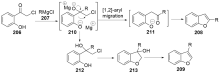
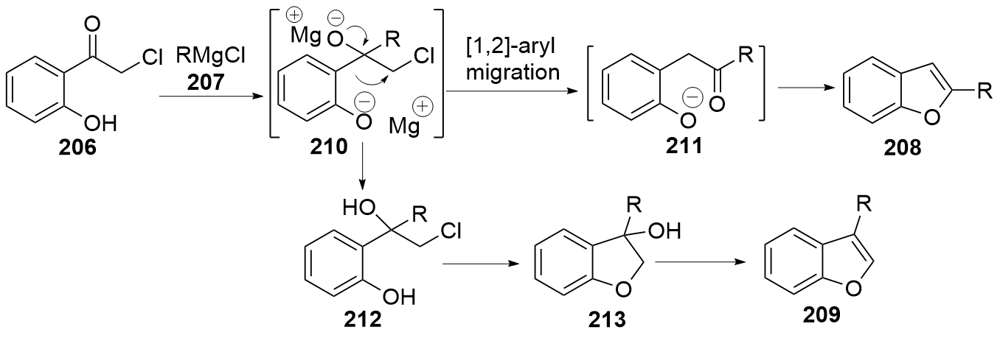




| [1] |
Miao, Y. H.; Hu, Y. H.; Yang, J.; Liu, T.; Sun, J.; Wang, X. J. RSC Adv. 2019, 9, 27510.
doi: 10.1039/C9RA04917G |
| [2] |
Karagöz, A. Ç.; Reiter, C.; Seo, E. J. Bioorg. Med. Chem. 2018, 26, 3610.
doi: 10.1016/j.bmc.2018.05.041 |
| [3] |
Xu, X. L.; Yang, Y. R.; Mo, X. F.; Wei, J. L.; Zhang, X. J.; You, Q. D. Eur. J. Med. Chem. 2017, 137, 45.
doi: 10.1016/j.ejmech.2017.05.042 |
| [4] |
Thévenin, M.; Thoret, S.; Grellier, P.; Dubois, J. Bioorg. Med. Chem. 2013, 21, 4885.
doi: 10.1016/j.bmc.2013.07.002 |
| [5] |
(a) Liang, Z.; Xu, H.; Tian, Y.; Guo, M.; Su, X.; Guo, C. Molecules 2016, 21, 732.
doi: 10.3390/molecules21060732 |
|
(b) Kenchappa, R.; Bodke, Y. D.; Telkar, S.; Sindhe, M. A. J. Chem. Biol. 2017, 10, 1.
|
|
| [6] |
Tan, Y. X.; Gong, T.; Liu, C. Chem. Pharm. Bull. 2010, 58, 579.
doi: 10.1248/cpb.58.579 |
| [7] |
Aswathanarayanappa, C.; Bheemappa, E.; Bodke, Y. D.; Bhovi, V. K.; Ningegowda, R.; Shivakumar, M. C. Med. Chem. Res. 2012, 22, 78.
doi: 10.1007/s00044-012-0017-y |
| [8] |
(a) Schroeter, F.; Soellner, J.; Thomas Strassner, T. ACS Catal. 2017, 7, 3004.
doi: 10.1021/acscatal.6b03655 |
|
(b) Hamasaka, G.; Roy, D.; Tazawa, A.; Uozumi, Y. ACS Catal. 2019, 9, 11640.
doi: 10.1021/acscatal.9b04593 |
|
|
(c) Chen, Z. K.; Wang, B. J.; Zhang, J. T.; Yu, W. L.; Liu, Z. X.; Zhang, Y. H. Org. Chem. Front. 2015, 2, 1107.
doi: 10.1039/C5QO00004A |
|
| [9] |
Liu, Y.; Yao, B.; Deng, C. L.; Tang, R. Y.; Zhang, X. G.; Li, J. H. Org. Lett. 2011, 13, 1126.
doi: 10.1021/ol1031552 |
| [10] |
Geary, L. M.; Hultin, P. G. Eur. J. Org. Chem. 2010, 2010, 5563.
|
| [11] |
Liu, J.; Chen, W.; Ji, Y.; Wang, L. Adv. Synth. Catal. 2012, 354, 1585.
doi: 10.1002/adsc.201100875 |
| [12] |
Rehan, M.; Nallagonda, R.; Das, B. G.; Meena, T.; Ghorai, P. J. Org. Chem. 2017, 82, 3411.
doi: 10.1021/acs.joc.6b02752 |
| [13] |
Yamaguchi, M.; Ozawa, H.; Katsumata, H.; Akiyama, T.; Manabe, K. Tetrahedron Lett. 2018, 59, 3175.
|
| [14] |
Zhou, L. X.; Shi, Y. X.; Zhu, X. Y.; Zhang, P. Tetrahedron Lett. 2019, 60, 2005.
|
| [15] |
Seo, J.; Chen, C. W.; Lee, W. Y.; Jeon, J. E.; Chen, P. L.; Chuang, S. C.; Joo, J. M. Synthesis 2021, 53, 3001.
doi: 10.1055/a-1502-3641 |
| [16] |
Sun, L. L.; Liao, Z. Y.; Tang, R. Y.; Deng, C. L.; Zhang, X. G. J. Org. Chem. 2012, 77, 2850.
doi: 10.1021/jo3000404 |
|
(b) Komiyama, M.; Tsuchiya, H.; Teramoto, M.; Yajima, N.; Kurokawa, M.; Minamizono, K.; Tsuchiya, N.; Kato, Y.; Sato, Y.; Dohi, M. Org. Process Res. Dev. 2018, 22, 1306.
doi: 10.1021/acs.oprd.8b00164 |
|
| [17] |
Nagamochi, M.; Fang, Y. Q.; Lautens, M. Org. Lett. 2007, 9, 2955.
doi: 10.1021/ol071370w |
| [18] |
Lakshminarayana, B.; Chakraborty, J.; Satyanarayana, G.; Subrahmanyam, C. RSC Adv. 2018, 8, 21030.
doi: 10.1039/C8RA03697G |
| [19] |
Cheng, L. J.; Mankad, N. P. Chem. Soc. Rev. 2020, 49, 8036.
doi: 10.1039/D0CS00316F |
| [20] |
Newman, S. G.; Aureggi, V.; Bryan, C. S.; Lautens, M. Chem. Commun. 2009, 45, 5236.
|
| [21] |
Zhou, L.; Shi, Y.; Xiao, Q.; Liu, Y. Z.; Ye, F.; Zhang, Y.; Wang, J. B. Org. Lett. 2011, 13, 968.
doi: 10.1021/ol103009n pmid: 21268646 |
| [22] |
Hirano, K.; Satoh, T.; Miura, M. Org. Lett. 2011, 13, 2395.
doi: 10.1021/ol200651r pmid: 21449554 |
| [23] |
Liu, J. M.; Zhang, N. F.; Yue, Y. Y.; Wang, D.; Zhang, Y. L.; Zhang, X.; Zhou, K. L. RSC Adv. 2013, 3, 3865.
doi: 10.1039/c3ra00093a |
| [24] |
Liu, Y.; Wang, H.; Wan, J. P. J. Org. Chem. 2014, 79, 10599.
doi: 10.1021/jo5017508 |
| [25] |
Gao, D.; Back, T. G. Chem.-Eur. J. 2012, 18, 14828.
doi: 10.1002/chem.201202307 |
| [26] |
Fu, R. G.; Li, Z. Org. Lett. 2018, 20, 2342.
doi: 10.1021/acs.orglett.8b00676 |
| [27] |
Villuri, B. K.; Ichake, S. S.; Reddy, S. R.; Kavala, V.; Bandi, V.; Kuo, C. W.; Yao, C. F. J. Org. Chem. 2018, 83, 10241.
doi: 10.1021/acs.joc.8b01443 |
| [28] |
Zhu, X. R.; Deng, C. L. Synthesis 2021, 1842.
|
| [29] |
(a) Krause, N.; Winter, C. Chem. Rev. 2011, 111, 1994.
doi: 10.1021/cr1004088 pmid: 21391565 |
|
(b) Corma, A.; Leyva-Pérez, A.; Sabater, M. J. Chem. Rev. 2011, 111, 1657.
doi: 10.1021/cr100414u pmid: 21391565 |
|
| [30] |
Fernandez-Canelas, P.; Rubio, E.; Gonzalez, J. M. Org. Lett. 2019, 21, 6566.
doi: 10.1021/acs.orglett.9b02551 |
| [31] |
(a) Achar, T. K.; Maiti, S.; Jana, S.; Debabrata Maiti, D. ACS Catal. 2020, 10, 13748.
doi: 10.1021/acscatal.0c03743 |
|
(b) Masahiro Murakami, M.; Ishida, N. Chem. Rev. 2021, 121, 264.
doi: 10.1021/acs.chemrev.0c00569 |
|
| [32] |
Hu, Z. Y.; Tong, X. F.; Liu, G. X. Org. Lett. 2016, 18, 2058.
doi: 10.1021/acs.orglett.6b00689 |
| [33] |
Sun, P.; Gao, S.; Yang, C.; Guo, S. J.; Lin, A. J.; Yao, H. Q. Org. Lett. 2016, 18, 6464.
doi: 10.1021/acs.orglett.6b03355 |
| [34] |
Shi, Z.; Schröder, N.; Glorius, F. Angew. Chem., Int. Ed. 2012, 51, 8092.
doi: 10.1002/anie.201203224 |
| [35] |
Trost, B. M.; Frederiksen, M. U.; Rudd, M. T. Angew. Chem., Int. Ed. 2005, 44, 6630.
doi: 10.1002/anie.200500136 |
| [36] |
Varela-Fernández, A.; González-Rodríguez, C.; Varela, J. A.; Castedo, L.; Saá, C. Org. Lett. 2009, 11, 5350.
doi: 10.1021/ol902212h pmid: 19860408 |
| [37] |
Lee, D. H.; Kwon, K. H.; Yi, C. S. J. Am. Chem. Soc. 2012, 134, 7325.
doi: 10.1021/ja302710v |
| [38] |
(a) Suzuki, T. Chem. Rev. 2011, 111, 1825.
doi: 10.1021/cr100378r |
|
(b) Pan, S.; Shibata, T. ACS Catal. 2013, 3, 704.
doi: 10.1021/cs400066q |
|
| [39] |
Shibata, T.; Hashimoto, Y.-K.; Otsuka, M.; Tsuchikama, K.; Endo, K. Synlett 2011, 2075.
|
| [40] |
Shibata, T.; Hashimoto, Y.-k.; Otsuka, M.; Tsuchikama, K.; Endo, K. Synth. Catal. 2009, 351, 2850.
doi: 10.1002/adsc.200900570 |
| [41] |
Anxionnat, B.; Pardo, D. G.; Ricci, G.; Rossen, K.; Cossy, J. Org. Lett. 2013, 15, 3876.
doi: 10.1021/ol401610e pmid: 23859265 |
| [42] |
(a) Schneider, U.; Kobayashi, S. Acc. Chem. Res. 2012, 45, 1331.
doi: 10.1021/ar300008t |
|
(b) Du, G.; Wang, G.; Ma, W.; Yang, Q.; Bao, W.; Liang, X.; Zhu, L.; Lee, C.-S. Synlett 2017, 28, 1394.
doi: 10.1055/s-0036-1588777 |
|
| [43] |
Alonso-Maranon, L.; Martinez, M. M.; Sarandeses, L. A.; Gomez-Bengoa, E.; Perez Sestelo, J. I. J. Org. Chem. 2018, 83, 7970.
doi: 10.1021/acs.joc.8b00829 |
| [44] |
Radadiya, A.; Shah, A. Eur. J. Med. Chem. 2015, 97, 356.
doi: 10.1016/j.ejmech.2015.01.021 pmid: 25703339 |
| [45] |
Donohoe, T. J.; Jones, C. R.; Winter, C.In Comprehensive Organic Synthesis, 2nd ed., Vol. 8, Eds.: Knochel, P.; Molander, G. A. Elsevier, Amsterdam, 2014, pp. 794-837.
|
| [46] |
Wu, F. T.; Bai, R.X.; Gu, Y. L. Adv. Synth. Catal. 2016, 358, 2307.
doi: 10.1002/adsc.201600048 |
| [47] |
Tomkinson, N. C. O.; Contiero, F.; Jones, K.; Matts, E.; Porzelle, A. Synlett 2009, 18, 3003.
|
| [48] |
Chen, W.; Zhang, Y. C.; Zhang, L.; Wang, M.; Wang, L. Chem Commun. 2011, 47, 10476.
doi: 10.1039/c1cc13967c |
| [49] |
Schevenels, F.; Markó, I. E. Org. Lett., 2012, 14, 1298.
doi: 10.1021/ol300186s pmid: 22356557 |
| [50] |
Xie, Y. S.; Kumar, D.; Bodduri, V. D. V.; Tarani, P. S.; Zhao, B. X.; Miao, J. Y.; Jang, K.; Shin, D. S. Tetrahedron Lett. 2014, 55, 2796.
doi: 10.1016/j.tetlet.2014.02.116 |
| [51] |
Kenchappa, R.; Bodke, Y. D.; Telkar, S.; Nagaraja, O. Russ. J. Gen. Chem. 2017, 87, 2027.
doi: 10.1134/S1070363217090195 |
| [52] |
Gouthami, P.; Chavan, L. N.; Chegondi, R.; Chandrasekhar, S. J. Org. Chem. 2018, 83, 3325.
doi: 10.1021/acs.joc.8b00360 |
| [53] |
Zhang, H. W.; Ma, C. M.; Zheng, Z. W.; Sun, R. W.; Yu, X. H.; Zhao, J. H. Chem. Commun. 2018, 54, 4935.
doi: 10.1039/C8CC02474J |
| [54] |
Ranjbari, M. A.; Tavakol, H. J. Org. Chem. 2021, 86, 4756.
doi: 10.1021/acs.joc.1c00143 |
| [55] |
Wirth, T.; Singh, F. V. Synthesis 2012, 44, 1171.
doi: 10.1055/s-0031-1290588 |
| [56] |
Singh, F. V.; Mangaonkar, S. Synthesis 2018, 50, 4940.
doi: 10.1055/s-0037-1610650 |
| [57] |
Pei, T.; Chen, C.-Y.; DiMichele, L.; Davies, I. W. Org. Lett. 2010, 12, 4972.
doi: 10.1021/ol102123u |
| [58] |
Rao, M. L. N.; Awasthi, D. K.; Talode, J. B. Tetrahedron Lett. 2012, 53, 2662.
doi: 10.1016/j.tetlet.2012.03.059 |
| [59] |
Wang. Z. W.; Li, Y. B.; Yan, B. Q.; Huang, M. M.; Wu, Y. J. Synlett 2015, 26, 531.
doi: 10.1055/s-0034-1379606 |
| [60] |
Wen, C.; Jiang, X.; Wu, K.; Luo, R. Q.; Li, Q. H. RSC Adv. 2020, 10, 19610.
doi: 10.1039/D0RA02984J |
| [61] |
Wen, C.; Wu, C.; Luo, R. Q.; Li, Q. H.; Chen, F. Synthesis 2021, 53, 3847.
doi: 10.1055/a-1516-8745 |
| [62] |
Mayhugh, A. L.; Luscombe, C. K. Org. Lett. 2021, 23, 7079.
doi: 10.1021/acs.orglett.1c02397 pmid: 34472874 |
| [63] |
Yorimitsu, H.; Baralle, A.; Otsuka, S.; Guérin, V.; Murakami, K.; Osuka, A. Synlett 2015, 26, 327.
doi: 10.1055/s-0034-1378914 |
| [64] |
Yoon. H. J.; Lee, Y. M. J. Org. Chem. 2015, 80, 10244.
doi: 10.1021/acs.joc.5b01863 |
| [65] |
Yang, Q.; He, Y.; Wang, T.; Zeng, L. Y.; Zhang, Z. T. Mol. Diversity 2016, 20, 9.
doi: 10.1007/s11030-015-9620-4 |
| [1] | Xiaoying Jia, Jiaxia Pu, Lirong Han, Qinghan Li. Research Progress in the Synthesis of Benzo[d]pentamembered Heterocyclic Thioethers Containing Two Heteroatoms [J]. Chinese Journal of Organic Chemistry, 2024, 44(1): 18-40. |
| [2] | Jiaxia Pu, Xiaoying Jia, Lirong Han, Qinghan Li. Research Progress of Visible Light Promoted C—N Bond Fracture to Construct C—C Bond [J]. Chinese Journal of Organic Chemistry, 2023, 43(8): 2591-2613. |
| [3] | Jingpeng Li, Shuntao Huang, Qi Yang, Weiqiang Li, Teng Liu, Chao Huang. A Practical Synthesis of (Z)-N-Vinyl Substituted N,O-Acetals under Continuous Flow Technology [J]. Chinese Journal of Organic Chemistry, 2023, 43(4): 1550-1558. |
| [4] | Nan Jiang, Guanjie Huang, Yan Hu, Bo Wang. Ruthenium-Catalyzed C—H [4+2] Annulation of Quinazolinones with Vinylene Carbonate [J]. Chinese Journal of Organic Chemistry, 2023, 43(4): 1537-1549. |
| [5] | Baichuan Mo, Chunxia Chen, Jinsong Peng. Research Progress in Application of Lignin and Its Derivatives Supported Metal Catalysts in Organic Synthesis [J]. Chinese Journal of Organic Chemistry, 2023, 43(4): 1215-1240. |
| [6] | Zujia Lu, Jian Qin, Jinting Wu, Wenli Cao, Baolong Kuang, Jianguo Zhang. Advances in the Synthesis of Energetic Compounds Based on 1,2,3-Triazoles [J]. Chinese Journal of Organic Chemistry, 2023, 43(2): 526-554. |
| [7] | Menghan Shen, Laiqiang Li, Quan Zhou, Jiehui Wang, Lei Wang. Visible-Light-Induced Regio-selective Oxidative Coupling of Quinoxalinones with Pyrrole Derivatives [J]. Chinese Journal of Organic Chemistry, 2023, 43(2): 697-704. |
| [8] | Peng Liu, Fuming Zhong, Lihao Liao, Weiqiang Tan, Xiaodan Zhao. Progress in the Construction of Spirocyclohexadienones via Alkyne-Involving Dearomatization [J]. Chinese Journal of Organic Chemistry, 2023, 43(12): 4019-4035. |
| [9] | Sining Qin. Research Progress in C—S Coupling Reactions of Aryl Halides [J]. Chinese Journal of Organic Chemistry, 2023, 43(11): 3761-3783. |
| [10] | Wei-Yuan Ma, Huifang Dai, Shaolin Kang, Tianlin Zhang, Xing-Zhong Shu. Hiyama Cross-Coupling Reaction of Aryl Vinylsilanes and Aryl Halides [J]. Chinese Journal of Organic Chemistry, 2023, 43(10): 3614-3622. |
| [11] | Tian Sang, Fan Jia, Jing He, Chuntian Li, Yan Liu, Ping Liu. I2-Catalyzed Cyclization of β-Ketonitrile with 1H-Pyrazol-5-amine [J]. Chinese Journal of Organic Chemistry, 2023, 43(1): 195-201. |
| [12] | Donghan Liu, Xihang Lu, Zhangmengjie Chai, Haoqi Yang, Yulin Sun, Fuchao Yu. Progress in Construction of 2H-Pyrrol-2-ones Skeleton [J]. Chinese Journal of Organic Chemistry, 2023, 43(1): 57-73. |
| [13] | Chuanchuan Wang, Zhiwei Ma, Xuehui Hou, Longhua Yang, Yajing Chen. Research and Application of N-Ts Cyanamides in Organic Synthesis [J]. Chinese Journal of Organic Chemistry, 2023, 43(1): 74-93. |
| [14] | Keli Wang, Jing Huang, Wei Liu, Zhilin Wu, Xianyong Yu, Jun Jiang, Weimin He. Direct Synthesis of 3-Sulfonylquinolines from N-Propargylanilines with Sulfonyl Chlorides [J]. Chinese Journal of Organic Chemistry, 2022, 42(8): 2527-2534. |
| [15] | Can Yong, Yun Li, Tao Bi, Guofeng Chen, Dongxia Zheng, Zhouyu Wang, Yuanyuan Zhang. Research Progress on the Synthesis and Activity of D-Galactose Derived Small Galectin Inhibitors [J]. Chinese Journal of Organic Chemistry, 2022, 42(5): 1307-1325. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||