Chinese Journal of Organic Chemistry ›› 2022, Vol. 42 ›› Issue (4): 965-977.DOI: 10.6023/cjoc202110040 Previous Articles Next Articles
REVIEWS
收稿日期:2021-10-28
修回日期:2021-11-29
发布日期:2021-12-22
通讯作者:
刘振
基金资助:
Youcai Zhu, Xinxin Ding, Li Sun, Zhen Liu( )
)
Received:2021-10-28
Revised:2021-11-29
Published:2021-12-22
Contact:
Zhen Liu
Supported by:Share
Youcai Zhu, Xinxin Ding, Li Sun, Zhen Liu. Advances in the Production of Acrylic Acid and Its Derivatives by CO2/C2H4 Coupling[J]. Chinese Journal of Organic Chemistry, 2022, 42(4): 965-977.
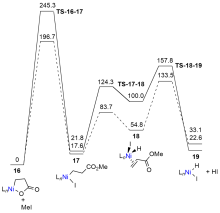
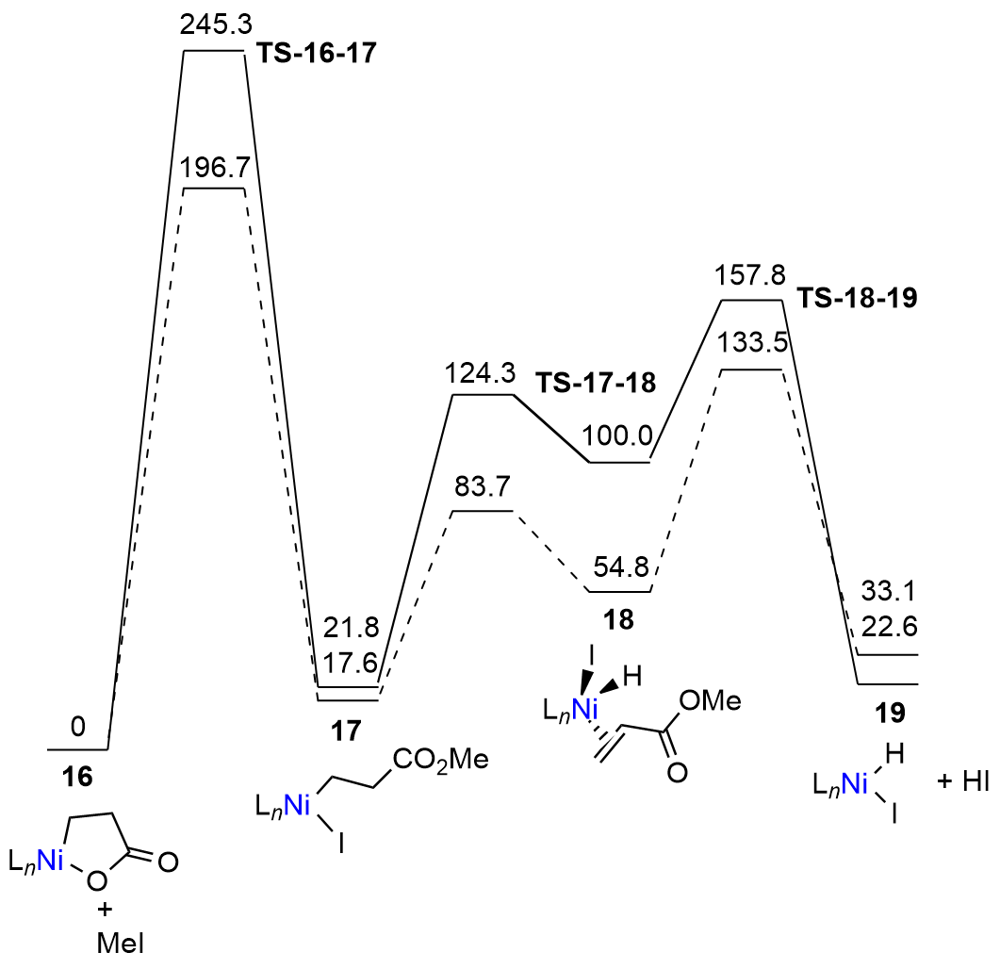
| Entry | Base | Additive | Time/h | Temp./℃ | Yield/% |
|---|---|---|---|---|---|
| 1 | NaOtBu | — | 0.25 | 25 | 90 |
| 2 | NaHMDS | — | 0.25 | 25 | 87 |
| 3 | NaOMe | — | 24 | 50 | 51 (71)a |
| 4 | NaOH | — | 24 | 50 | 0 (70)b |
| 5 | NaOPh | — | 72 | 70 | 0 |
| 6 | NBu4OMe | — | 72 | 50 | 0 |
| 7 | NBu4OMe | NaOArF | 72 | 50 | 47 |
| 8 | DBU | — | 72 | 70 | 0 |
| 9 | DBU | NaOArF | 72 | 70 | 0 |
| 10 | P1 | — | 72 | 50 | 0 |
| 11 | P1 | NaOArF | 72 | 50 | 40 |
| Entry | Base | Additive | Time/h | Temp./℃ | Yield/% |
|---|---|---|---|---|---|
| 1 | NaOtBu | — | 0.25 | 25 | 90 |
| 2 | NaHMDS | — | 0.25 | 25 | 87 |
| 3 | NaOMe | — | 24 | 50 | 51 (71)a |
| 4 | NaOH | — | 24 | 50 | 0 (70)b |
| 5 | NaOPh | — | 72 | 70 | 0 |
| 6 | NBu4OMe | — | 72 | 50 | 0 |
| 7 | NBu4OMe | NaOArF | 72 | 50 | 47 |
| 8 | DBU | — | 72 | 70 | 0 |
| 9 | DBU | NaOArF | 72 | 70 | 0 |
| 10 | P1 | — | 72 | 50 | 0 |
| 11 | P1 | NaOArF | 72 | 50 | 40 |
| Entrya | Ligand | TONc | Pd leaching/(mg•L–1) |
|---|---|---|---|
| 1b | none | 0 | n.d.d |
| 2 | PCy3 | 0 | <1 |
| 3 | dcpm | 0 | 13 |
| 4 | dcpe | 106 | 1 |
| 5 | dcpp | 22 | <1 |
| 6 | dcpb | 9 | <1 |
| 7 | dppe | 0 | <1 |
| 8 | dmpe | 0 | 49 |
| 9 | dtbpe | 17 | 14 |
| Entrya | Ligand | TONc | Pd leaching/(mg•L–1) |
|---|---|---|---|
| 1b | none | 0 | n.d.d |
| 2 | PCy3 | 0 | <1 |
| 3 | dcpm | 0 | 13 |
| 4 | dcpe | 106 | 1 |
| 5 | dcpp | 22 | <1 |
| 6 | dcpb | 9 | <1 |
| 7 | dppe | 0 | <1 |
| 8 | dmpe | 0 | 49 |
| 9 | dtbpe | 17 | 14 |
| [1] |
Park, C.; Lee, Y. T.; Lee, S. H. Atmos. Environ. 2021, 252, 118340.
doi: 10.1016/j.atmosenv.2021.118340 |
| [2] |
Cokoja, M.; Bruckmeier, C.; Rieger, B.; Herrmann, W. A.; Kühn, F. E. Angew. Chem., nt. Ed. 2011, 50, 8510.
|
| [3] |
Mikkelsen, M.; Jorgensen, M.; Krebs, F. C. Energy Environ. Sci. 2010, 3, 43.
doi: 10.1039/B912904A |
| [4] |
Aresta, M.; Dibenedetto, A.; Angelini, A. Chem. Rev. (Washington, DC, U. S.) 2014, 114, 1709.
doi: 10.1021/cr4002758 |
| [5] |
Yi, Y. P.; Hang, W.; Xi, C. J. Chin. J. Org. Chem. 2021, 41, 80. (in Chinese)
doi: 10.6023/cjoc202007013 |
|
( 易雅平, 杭炜, 席婵娟, 有机化学, 2021, 41, 80.)
doi: 10.6023/cjoc202007013 |
|
| [6] |
Liang, B, L.; Duan, H, M.; Hou, B, L.; Su, X.; Huang, Y, Q.; Wang, A, Q.; Wang, X, D.; Zhang, T. Chem. Ind. Eng. Prog. 2015, 34, 3746. (in Chinese)
|
|
( 梁兵连, 段洪敏, 侯宝林, 苏雄, 黄延强, 王爱琴, 王晓东, 张涛, 化工进展, 2015, 34, 3746.)
|
|
| [7] |
Zhang, W. Z.; Zhang, N.; Guo, X. C.; Lu, X. B. Chin. J. Org. Chem. 2017, 37, 1309. (in Chinese)
doi: 10.6023/cjoc201701031 |
|
( 张文珍; 张宁; 郭春晓; 吕小兵, 有机化学, 2017, 37, 1309.)
|
|
| [8] |
Chen, K. H.; Li, H. R.; He, L. N. Chin. J. Org. Chem. 2020, 40, 2195. (in Chinese)
doi: 10.6023/cjoc202004030 |
|
( 陈凯宏, 李红茹, 何良年, 有机化学, 2020, 40, 2195.)
doi: 10.6023/cjoc202004030 |
|
| [9] |
Zhang, Y.-G.; Riduan, S. N. Angew. Chem., Int. Ed. 2011, 50, 6210.
doi: 10.1002/anie.201101341 |
| [10] |
Sakakura, T.; Choi, J.-C.; Yasuda, H. Chem. Rev. (Washington, DC, U. S.) 2007, 107, 2365.
doi: 10.1021/cr068357u |
| [11] |
Tortajada, A.; Julia-Hernandez, F.; Boerjesson, M.; Moragas, T.; Martin, R. Angew. Chem., Int. Ed. 2018, 57, 15948.
doi: 10.1002/anie.201803186 |
| [12] |
Xu, P.; Wang, S. Y.; Fang, Y.; Ji, S. J. Chin. J. Org. Chem. 2018, 38, 1626. (in Chinese)
doi: 10.6023/cjoc201801046 |
|
( 徐佩, 汪顺义, 方毅, 纪顺俊, 有机化学, 2018, 38, 1626.)
doi: 10.6023/cjoc201801046 |
|
| [13] |
Tappe, N. A.; Reich, R. M.; D'Elia, V.; Kühn, F. E. Dalton Trans. 2018, 47, 13281.
doi: 10.1039/C8DT02346H |
| [14] |
Anthofer, M. H.; Wilhelm, M. E.; Cokoja, M.; Markovits, I. I. E.; Poethig, A.; Mink, J.; Herrmann, W. A.; Kühn, F. E. Catal. Sci. Technol. 2014, 4, 1749.
doi: 10.1039/c3cy01024d |
| [15] |
Dutta, B.; Sofack-Kreutzer, J.; Ghani, A. A.; D'Elia, V.; Pelletier, J. D. A.; Cokoja, M.; Kühn, F. E.; Basset, J.-M. Catal. Sci. Technol. 2014, 4, 1534.
doi: 10.1039/C4CY00003J |
| [16] |
Kissling, S.; Altenbuchner, P. T.; Lehenmeier, M. W.; Herdtweck, E.; Deglmann, P.; Seemann, U. B.; Rieger, B. Chem.-Eur. J. 2015, 21, 8148.
doi: 10.1002/chem.201406055 pmid: 25900151 |
| [17] |
Gao, P.; Cui, X.; Zhong, L, S.; Sun, Y, H.. Chem. Ind. Eng. Prog. 2019, 38, 183. (in Chinese)
|
|
( 高鹏, 崔勖, 钟良枢, 孙予罕, 化工进展, 2019, 38, 183.)
|
|
| [18] |
Li, J.; Deng, Y, Y.; Yang, L.; Cao, X. J. Chem. Ind. Eng. Prog. 2013, 32, 340. (in Chinese)
|
|
( 李静, 邓廷云, 杨林, 曹建新, 化工进展, 2013, 32, 340.)
|
|
| [19] |
Sasaki, Y.; Inoue, Y.; Hashimoto, H. J. Chem. Soc., Chem. Commun. 1976, 605.
|
| [20] |
Inoue, Y.; Sasaki, Y.; Hashimoto, H. Bull. Chem. Soc. Jpn. 1978, 51, 2375.
doi: 10.1246/bcsj.51.2375 |
| [21] |
Lejkowski, M. L.; Lindner, R.; Kageyama, T.; Bodizs, G. E.; Plessow, P. N.; Mueller, I. B.; Schaefer, A.; Rominger, F.; Hofmann, P.; Futter, C.; Schunk, S. A.; Limbach, M. Chem.-Eur. J. 2012, 18, 14017.
doi: 10.1002/chem.201201757 pmid: 22996190 |
| [22] |
Brill, M.; Lazreg, F.; Cazin, C. S. J.; Nolan, S. P. Top. Organomet. Chem. 2016, 53, 225.
|
| [23] |
Hendriksen, C.; Pidko, E. A.; Yang, G.; Schaeffner, B.; Vogt, D. Chem.-Eur. J. 2014, 20, 12037.
doi: 10.1002/chem.201404082 pmid: 25116123 |
| [24] |
Zhang, Z.; Guo, F.; Kühn, F. E.; Sun, J.; Zhou, M.; Fang, X. Appl. Organomet. Chem. 2017, 31, e3567.
doi: 10.1002/aoc.3567 |
| [25] |
Hollering, M.; Dutta, B.; Kühn, F. E. Coord. Chem. Rev. 2016, 309, 51.
doi: 10.1016/j.ccr.2015.10.002 |
| [26] |
Wang, X.; Wang, H.; Sun, Y. Chem 2017, 3, 211.
doi: 10.1016/j.chempr.2017.07.006 |
| [27] |
Schaub, T. Top. Organomet. Chem. 2019, 65, 253.
|
| [28] |
Lee, S. Y. T.; Ghani, A. A.; D'Elia, V.; Cokoja, M.; Herrmann, W. A.; Basset, J.-M.; Kühn, F. E. New J. Chem. 2013, 37, 3512.
doi: 10.1039/c3nj00693j |
| [29] |
Kunihiro, K.; Heyte, S.; Paul, S.; Roisnel, T.; Carpentier, J.-F.; Kirillov, E. Chem.-Eur. J. 2021, 27, 3997.
doi: 10.1002/chem.202005083 pmid: 33378130 |
| [30] |
Kraus, S.; Rieger, B. Top. Organomet. Chem. 2016, 53, 199.
|
| [31] |
Tsuji, Y.; Fujihara, T. Chem. Commun. (Cambridge, U. K.) 2012, 48, 9956.
doi: 10.1039/c2cc33848c |
| [32] |
Hoberg, H.; Schaefer, D. J. Organomet. Chem. 1983, 251, C51.
doi: 10.1016/S0022-328X(00)98789-8 |
| [33] |
Papai, I.; Schubert, G.; Mayer, I.; Besenyei, G.; Aresta, M. Organometallics 2004, 23, 5252.
doi: 10.1021/om049496+ |
| [34] |
Dreissig, W.; Dietrich, H. Acta Crystallogr., Sect. B: Struct. Sci., Cryst. Eng. Mater. 1981, B37, 931.
|
| [35] |
Fischer, R.; Langer, J.; Malassa, A.; Walther, D.; Goerls, H.; Vaughan, G. Chem. Commun. (Cambridge, U. K.) 2006, 2510.
|
| [36] |
Graham, D. C.; Mitchell, C.; Bruce, M. I.; Metha, G. F.; Bowie, J. H.; Buntine, M. A. Organometallics 2007, 26, 6784.
doi: 10.1021/om700592w |
| [37] |
Li, Y.; Liu, Z.; Cheng, R.; Liu, B. ChemCatChem 2018, 10, 1420.
doi: 10.1002/cctc.201701763 |
| [38] |
Al-Ghamdi, M.; Vummaleti, S. V. C.; Falivene, L.; Pasha, F. A.; Beetstra, D. J.; Cavallo, L. Organometallics 2017, 36, 1107.
doi: 10.1021/acs.organomet.6b00905 |
| [39] |
Aresta, M.; Pastore, C.; Giannoccaro, P.; Kovacs, G.; Dibenedetto, A.; Papai, I. Chem.-Eur. J. 2007, 13, 9028.
doi: 10.1002/chem.200700532 |
| [40] |
Bruckmeier, C.; Lehenmeier, M. W.; Reichardt, R.; Vagin, S.; Rieger, B. Organometallics 2010, 29, 2199.
doi: 10.1021/om100060y |
| [41] |
Lee, S. Y. T.; Cokoja, M.; Drees, M.; Li, Y.; Mink, J.; Herrmann, W. A.; Kühn, F. E. ChemSusChem 2011, 4, 1275.
doi: 10.1002/cssc.201000445 |
| [42] |
Liang, L.-C.; Chien, P.-S.; Lee, P.-Y. Organometallics 2008, 27, 3082.
doi: 10.1021/om701294a |
| [43] |
Guo, W.; Michel, C.; Schwiedernoch, R.; Wischert, R.; Xu, X.; Sautet, P. Organometallics 2014, 33, 6369.
doi: 10.1021/om5006808 |
| [44] |
Plessow, P. N.; Weigel, L.; Lindner, R.; Schaefer, A.; Rominger, F.; Limbach, M.; Hofmann, P. Organometallics 2013, 32, 3327.
doi: 10.1021/om400262b |
| [45] |
Jin, D.; Williard, P. G.; Hazari, N.; Bernskoetter, W. H. Chem.-Eur. J. 2014, 20, 3205.
doi: 10.1002/chem.201304196 |
| [46] |
Huguet, N.; Jevtovikj, I.; Gordillo, A.; Lejkowski, M. L.; Lindner, R.; Bru, M.; Khalimon, A. Y.; Rominger, F.; Schunk, S. A.; Hofmann, P.; Limbach, M. Chem.-Eur. J. 2014, 20, 16858.
doi: 10.1002/chem.201405528 pmid: 25359188 |
| [47] |
Knopf, I.; Tofan, D.; Beetstra, D.; Al-Nezari, A.; Al-Bahily, K.; Cummins, C. C. Chem. Sci. 2017, 8, 1463.
doi: 10.1039/C6SC03614G |
| [48] |
Li, Y.; Liu, Z.; Zhang, J.; Cheng, R.; Liu, B. ChemCatChem 2018, 10, 5669.
doi: 10.1002/cctc.201801305 |
| [49] |
Hopkins, M. N.; Shimmei, K.; Uttley, K. B.; Bernskoetter, W. H. Organometallics 2018, 37, 3573.
doi: 10.1021/acs.organomet.8b00260 |
| [50] |
Uttley, K. B.; Shimmei, K.; Bernskoetter, W. H. Organometallics 2020, 39, 1573.
doi: 10.1021/acs.organomet.9b00708 |
| [51] |
Musco, A.; Perego, C.; Tartiari, V. Inorg. Chim. Acta 1978, 28, L147.
doi: 10.1016/S0020-1693(00)87385-5 |
| [52] |
Langer, J.; Fischer, R.; Goerls, H.; Walther, D. Eur. J. Inorg. Chem. 2007, 2257.
|
| [53] |
Stieber, S. C. E.; Huguet, N.; Kageyama, T.; Jevtovikj, I.; Ariyananda, P.; Gordillo, A.; Schunk, S. A.; Rominger, F.; Hofmann, P.; Limbach, M. Chem. Commun. (Cambridge, U. K.) 2015, 51, 10907.
doi: 10.1039/C5CC01932J |
| [54] |
Manzini, S.; Huguet, N.; Trapp, O.; Schaub, T. Eur. J. Org. Chem. 2015, 2015, 7122.
doi: 10.1002/ejoc.201501113 |
| [55] |
Manzini, S.; Cadu, A.; Schmidt, A.-C.; Huguet, N.; Trapp, O.; Paciello, R.; Schaub, T. ChemCatChem 2017, 9, 2269.
doi: 10.1002/cctc.201601150 |
| [56] |
Li, B.; Kyran, S. J.; Yeung, A. D.; Bengali, A. A.; Darensbourg, D. J. Inorg. Chem. 2013, 52, 5438.
doi: 10.1021/ic4003737 |
| [57] |
Ito, T.; Takahashi, K.; Iwasawa, N. Organometallics 2019, 38, 205.
doi: 10.1021/acs.organomet.8b00789 |
| [58] |
Takahashi, K.; Hirataka, Y.; Ito, T.; Iwasawa, N. Organometallics 2020, 39, 1561.
doi: 10.1021/acs.organomet.9b00659 |
| [59] |
Hoberg, H.; Jenni, K.; Angermund, K.; Krüger, C. Angew. Chem., Int. Ed. 1987, 26, 153.
|
| [60] |
Hanna, B. S.; MacIntosh, A. D.; Ahn, S.; Tyler, B. T.; Palmore, G. T. R.; Williard, P. G.; Bernskoetter, W. H. Organometallics 2014, 33, 3425.
doi: 10.1021/om500324h |
| [61] |
Alvarez, R.; Carmona, E.; Galindo, A.; Gutierrez, E.; Marin, J. M.; Monge, A.; Poveda, M. L.; Ruiz, C.; Savariault, J. M. Organometallics 1989, 8, 2430.
doi: 10.1021/om00112a026 |
| [62] |
Alvarez, R.; Carmona, E.; Cole-Hamilton, D. J.; Galindo, A.; Gutierrez-Puebla, E.; Monge, A.; Poveda, M. L.; Ruiz, C. J. Am. Chem. Soc. 1985, 107, 5529.
doi: 10.1021/ja00305a037 |
| [63] |
Bernskoetter, W. H.; Tyler, B. T. Organometallics 2011, 30, 520.
doi: 10.1021/om100891m |
| [64] |
Knopf, I.; Courtemanche, M.-A.; Cummins, C. C. Organometallics 2017, 36, 4834.
doi: 10.1021/acs.organomet.7b00734 |
| [65] |
Yamashita, K.; Chatani, N. Synlett 2005, 919.
|
| [66] |
Aresta, M.; Quaranta, E. J. Organomet. Chem. 1993, 463, 215.
doi: 10.1016/0022-328X(93)83420-Z |
| [1] | Wenwen Chen, Qin Zhang, Songyue Zhang, Fangfang Huang, Xinyin Zhang, Jianfeng Jia. Visible Light Promoted Coupling Reaction of Alkynyl Iodide and Sodium Sulphinate without Photocatalyst [J]. Chinese Journal of Organic Chemistry, 2024, 44(2): 584-592. |
| [2] | Huanqing Li, Zhaohua Chen, Zujia Chen, Qiwen Qiu, Youcai Zhang, Sihong Chen, Zhaoyang Wang. Research Progress in Mercury Ion Fluorescence Probes Based on Organic Small Molecules [J]. Chinese Journal of Organic Chemistry, 2023, 43(9): 3067-3077. |
| [3] | Zujia Chen, Shiwei Yu, Yongjun Zhou, Huanqing Li, Qiwen Qiu, Miaoxin Li, Zhaoyang Wang. Application of BF3•OEt2 in Organic Synthesis as a Catalyst or Synthon [J]. Chinese Journal of Organic Chemistry, 2023, 43(9): 3107-3118. |
| [4] | Xu Liao, Zeyu Wang, Wufei Tang, Jinqing Lin. Progress in Porous Organic Polymer for Chemical Fixation of Carnbon Dioxide [J]. Chinese Journal of Organic Chemistry, 2023, 43(8): 2699-2710. |
| [5] | Zijie Song, Jun Liu, Ying Bai, Jiayun Li, Jiajian Peng. Progress in Catalysis Transformation of Carbon Dioxide through Hydrosilylation [J]. Chinese Journal of Organic Chemistry, 2023, 43(6): 2068-2080. |
| [6] | Xiaoyu Lu, Xiaomei Sun, Yaqing Niu, Junchao Wang, Wenjing Yin, Mengting Gao, Zi Liu, Zhenghuan Wei, Tinghua Tao. Copper-Catalyzed Decarboxylative Cross-Coupling of α‑Fluoroacrylic Acids with N-Tosyl Oxaziridines [J]. Chinese Journal of Organic Chemistry, 2023, 43(6): 2110-2119. |
| [7] | Cunjing Miao, Jiaqi Yao. Recent Advances in the Transformation Reactions of Aromatic Nitriles via C—CN Bond Cleavage [J]. Chinese Journal of Organic Chemistry, 2023, 43(4): 1341-1364. |
| [8] | Yongzhou Pan, Xiujin Meng, Yingchun Wang, Muxue He. Recent Progress in Electrochemical Fixation of CO2 to Construct Carboxylic Acid Derivatives [J]. Chinese Journal of Organic Chemistry, 2023, 43(4): 1416-1434. |
| [9] | Tingting Liu, Yucai Hu, An Shen. Mechanism of Carbon-Carbon Coupling Reactions Catalyzed by Imine-Ligand-Assisted N-Heterocyclic Carbene Palladium Complexes [J]. Chinese Journal of Organic Chemistry, 2023, 43(2): 622-628. |
| [10] | Guijie Liu, Zhengqiang Fu, Fei Chen, Caixia Xu, Min Li, Ning Liu. N-Heterocyclic Carbene-Pyridine Manganese Complex/ Tetrabutylammonium Iodide Catalyzed Synthesis of Cyclic Carbonate from CO2 and Epoxide [J]. Chinese Journal of Organic Chemistry, 2023, 43(2): 629-635. |
| [11] | Ning Liu, Xiaodan Cuan, Hui Li, Xiyan Duan. Progress in the Study of α-Functionalization of Enaminone [J]. Chinese Journal of Organic Chemistry, 2023, 43(2): 602-621. |
| [12] | Menghan Shen, Laiqiang Li, Quan Zhou, Jiehui Wang, Lei Wang. Visible-Light-Induced Regio-selective Oxidative Coupling of Quinoxalinones with Pyrrole Derivatives [J]. Chinese Journal of Organic Chemistry, 2023, 43(2): 697-704. |
| [13] | Yueling Liu, Xinxin Zhong, Ganbing Zhang. Density Functional Theory Study for Exploring the Mechanisms of the [3+2] Cycloaddition Reactions between 1-R-3-Phenylpropylidenecyclopropane (R=Me/H) and Furfural Catalyzed by Pd(0) [J]. Chinese Journal of Organic Chemistry, 2023, 43(2): 660-667. |
| [14] | Biao Ma, Miaomiao Zhang, Zhanyu Li, Jinsong Peng, Chunxia Chen. Recent Advance of Transition Metal-Free Catalyzed Suzuki-Type Cross Coupling Reaction [J]. Chinese Journal of Organic Chemistry, 2023, 43(2): 455-470. |
| [15] | Sining Qin. Research Progress in C—S Coupling Reactions of Aryl Halides [J]. Chinese Journal of Organic Chemistry, 2023, 43(11): 3761-3783. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||