Chinese Journal of Organic Chemistry ›› 2023, Vol. 43 ›› Issue (4): 1517-1524.DOI: 10.6023/cjoc202209018 Previous Articles Next Articles
Special Issue: 有机氟化学虚拟合辑
ARTICLES
收稿日期:2022-09-14
修回日期:2022-11-14
发布日期:2022-11-21
通讯作者:
郝健, 沈其龙
基金资助:
Jing Liua, Jian Haoa( ), Qilong Shenb(
), Qilong Shenb( )
)
Received:2022-09-14
Revised:2022-11-14
Published:2022-11-21
Contact:
Jian Hao, Qilong Shen
Supported by:Share
Jing Liu, Jian Hao, Qilong Shen. Visible-Light-Promoted Direct Trifluoromethylation of Tryptophan-Containing Oligapeptides with YlideFluor[J]. Chinese Journal of Organic Chemistry, 2023, 43(4): 1517-1524.
| Entry | Solvent | Base | Light | Time/h | Yield/% |
|---|---|---|---|---|---|
| 1 | DMSO | DBU | Blue | 12 | 60 |
| 2 | DMSO | DMAP | Blue | 12 | 27 |
| 3 | DMSO | DIPEA | Blue | 12 | 25 |
| 4 | DMSO | Cs2CO3 | Blue | 12 | 18 |
| 5 | DMSO | NaHCO3 | Blue | 12 | 41 |
| 6 | CH3CN | DBU | Blue | 12 | 29 |
| 7 | DMF | DBU | Blue | 12 | 28 |
| 8 | DMAc | DBU | Blue | 12 | 22 |
| 9 | NMP | DBU | Blue | 12 | 18 |
| 10 | Acetone | DBU | Blue | 12 | 46 |
| 11 | H2O | DBU | Blue | 12 | 33 |
| 12 | THF | DBU | blue | 12 | 2 |
| 13 | CH2Cl2 | DBU | Blue | 12 | 45 |
| 14 | DMSO | DBU | White | 12 | 54 |
| 15 | DMSO | DBU | Purple | 12 | 56 |
| 16 | DMSO | DBU | 12 | 46 | |
| 17 | DMSO | DBU | Blue | 6 | 54 |
| 18 | DMSO | DBU | Blue | 9 | 58 |
| 19 | DMSO | Blue | 12 | 34 | |
| 20c | DMSO | 12 |
| Entry | Solvent | Base | Light | Time/h | Yield/% |
|---|---|---|---|---|---|
| 1 | DMSO | DBU | Blue | 12 | 60 |
| 2 | DMSO | DMAP | Blue | 12 | 27 |
| 3 | DMSO | DIPEA | Blue | 12 | 25 |
| 4 | DMSO | Cs2CO3 | Blue | 12 | 18 |
| 5 | DMSO | NaHCO3 | Blue | 12 | 41 |
| 6 | CH3CN | DBU | Blue | 12 | 29 |
| 7 | DMF | DBU | Blue | 12 | 28 |
| 8 | DMAc | DBU | Blue | 12 | 22 |
| 9 | NMP | DBU | Blue | 12 | 18 |
| 10 | Acetone | DBU | Blue | 12 | 46 |
| 11 | H2O | DBU | Blue | 12 | 33 |
| 12 | THF | DBU | blue | 12 | 2 |
| 13 | CH2Cl2 | DBU | Blue | 12 | 45 |
| 14 | DMSO | DBU | White | 12 | 54 |
| 15 | DMSO | DBU | Purple | 12 | 56 |
| 16 | DMSO | DBU | 12 | 46 | |
| 17 | DMSO | DBU | Blue | 6 | 54 |
| 18 | DMSO | DBU | Blue | 9 | 58 |
| 19 | DMSO | Blue | 12 | 34 | |
| 20c | DMSO | 12 |
| Entry | Additive | Ylidefluor/equiv. | Yielda/% |
|---|---|---|---|
| 1 | TEMPO | 1.22 | 2 |
| 2 | Under air | 0.32 | 18 |
| 3 | BHT | 42 | |
| 4 | 1,4-Dinitrobenzene | 50 |
| Entry | Additive | Ylidefluor/equiv. | Yielda/% |
|---|---|---|---|
| 1 | TEMPO | 1.22 | 2 |
| 2 | Under air | 0.32 | 18 |
| 3 | BHT | 42 | |
| 4 | 1,4-Dinitrobenzene | 50 |

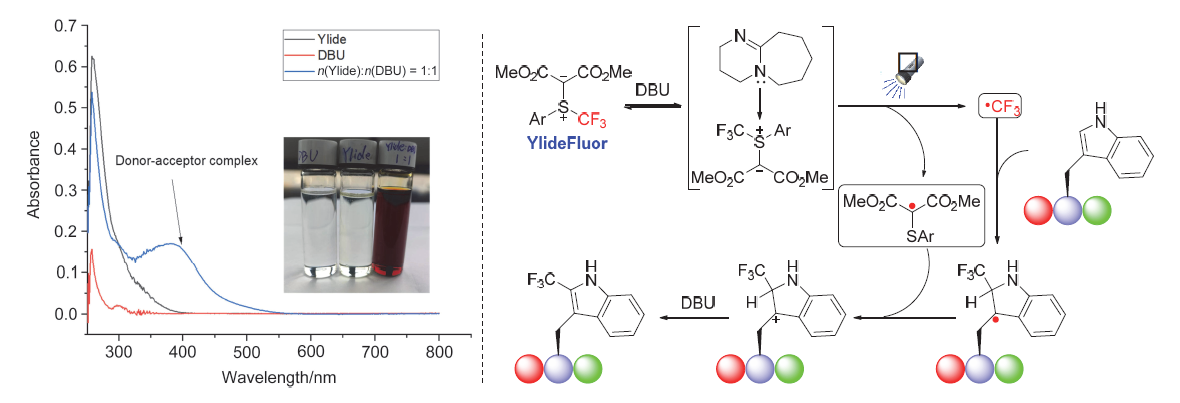
| [1] |
(a) Erak, M.; Bellmann-Sickert, K.; Els-Heindl, S.; Beck-Sickinger, A. G. Bioorg. Med. Chem. 2018, 26, 2759.
doi: 10.1016/j.bmc.2018.01.012 pmid: 31804810 |
|
(b) Kaspar, A. A.; Reichert, J. M. Drug Discovery Today 2013, 18, 807.
doi: 10.1016/j.drudis.2013.05.011 pmid: 31804810 |
|
|
(c) Malonis, R. J.; Lai, J. R.; Vergnolle, O. Chem. Rev. 2020, 120, 3210.
doi: 10.1021/acs.chemrev.9b00472 pmid: 31804810 |
|
| [2] |
(a) Reddy, B..Jow T.; Hantash, B. M. Exp. Dermatol. 2012, 21, 563.
doi: 10.1111/j.1600-0625.2012.01528.x |
|
(b) Ji, Y.-J.; Qiao, H.-Z.; He, J.-Y.; Li, W.-D.; Chen, R.; Wang, J.-J.; Wu, L.; Hu, R.-F.; Duan, J.-N.; Chen, Z.-P. J. Drug Target. 2017, 25, 597.
doi: 10.1080/1061186X.2017.1309044 |
|
| [3] |
deGruyter, J. N.; Malins, L. R.; Baran, P. S. Biochemistry 2017, 56, 3863.
doi: 10.1021/acs.biochem.7b00536 pmid: 28653834 |
| [4] |
Chalker, J. M.; Bernardes, G. J. L.; Lin, Y. A.; Davis, B. G. Chem. Asian J. 2009, 4, 630.
doi: 10.1002/asia.v4:5 |
| [5] |
(a) Ban, H.; Gavrilyuk, J.; Barbas III, C. F. J. Am. Chem. Soc. 2010, 132, 1523.
doi: 10.1021/ja909062q |
|
(b) Ban, H.; Nagano, M.; Gavrilyuk, J.; Hakamata, W.; Inokuma, T.; Barbas III, C. F. Bioconjugate Chem. 2013, 24, 520.
doi: 10.1021/bc300665t |
|
| [6] |
Ohata, J.; Minus, M. B.; Abernathy, M. E.; Ball, Z. T. J. Am. Chem. Soc. 2016, 138, 7472.
doi: 10.1021/jacs.6b03390 pmid: 27249339 |
| [7] |
(a) Ruiz-Rodriguez, J.; Albericio, F.; Lavilla, R. Chem.-Eur. J. 2010, 16, 1124.
doi: 10.1002/chem.200902676 pmid: 20013969 |
|
(b) Seki, Y.; Ishiyama, T.; Sasaki, D.; Abe, J.; Sohma, Y.; Oisaki, K.; Kanai, M. J. Am. Chem. Soc. 2016, 138, 10798.
doi: 10.1021/jacs.6b06692 pmid: 20013969 |
|
|
(c) Hansen, M. B.; Hubalek, F.; Skrydstrup, T.; Hoeg-Jensen, T. Chem.-Eur. J. 2016, 22, 1572.
doi: 10.1002/chem.201504462 pmid: 20013969 |
|
|
(d) Ruan, Z.; Sauermann, N.; Manoni, E.; Ackermann, L. Angew. Chem., Int. Ed. 2017, 56, 3172.
doi: 10.1002/anie.201611118 pmid: 20013969 |
|
| [8] |
Guerrero, I.; Correa, A. Asian J. Org. Chem. 2020, 9, 898.
doi: 10.1002/ajoc.v9.6 |
| [9] |
(a) Leo, A.; Hansch, C.; Elkins, D. Chem. Rev. 1971, 71, 525.
doi: 10.1021/cr60274a001 |
|
(b) Hansch, C.; Leo, A.; Taft, R. W. Chem. Rev. 1991, 91, 165.
doi: 10.1021/cr00002a004 |
|
|
(c) Qing, F.-L.; Liu, X.-Y.; Ma, J.-A.; Shen, Q.; Song, Q.-L.; Tang, P.-P. CCS Chem. 2022, 4, 2518.
doi: 10.31635/ccschem.022.202201935 |
|
| [10] |
Wang, Y.-X.; Wang, J.-H.; Li, G.-X..He G. ; Chen, G. Org. Lett. 2017, 19, 1442.
doi: 10.1021/acs.orglett.7b00375 |
| [11] |
Imiolek, M.; Karunanithy, G.; Ng, W.-L.; Baldwin, A. J.; Gourverneur, V.; Davis, B. G. J. Am. Chem. Soc. 2018, 140, 1568.
doi: 10.1021/jacs.7b10230 |
| [12] |
Ding, B.; Weng, Y.; Liu, Y.-Q.; Song, C.-L.; Yin, L.; Yuan, J.-F.; Ren, Y.-R.; Lei, A.-W.; Chiang, C.-W. Eur. J. Org. Chem. 2019, 7596.
|
| [13] |
Guerrero, I.; Correa, A. Org. Lett. 2020, 22, 1754.
doi: 10.1021/acs.orglett.0c00033 pmid: 32052977 |
| [14] |
Kee, C. W.; Tack, O.; Guibbal, F.; Wilson, T. C.; Iseneggar, P. G.; Imiolek, M.; Verhoog, S.; Tilby, M.; Boscutti, G.; Ashworth, S.; Chupin, J.; Kashani, R.; Poh, A. W. J.; Sosabowski, J. K.; Macholl, S.; Plisson, C.; Cornelissen, B.; Willis, M. C.; Passchier, J.; Davis, B. G.; Gouverneur, V. J. Am. Chem. Soc. 2020, 142, 1180.
doi: 10.1021/jacs.9b11709 |
| [15] |
Liu, Y.-F.; Shao, X.-X.; Lu, L.; Shen, Q. Org. Lett. 2015, 17, 2752.
doi: 10.1021/acs.orglett.5b01170 |
| [16] |
(a) Liu, Y.-F.; Ling, Y.-J.; Ge, H.-M.; Lu, L.; Shen, Q. Chin. J. Chem. 2021, 39, 1667.
doi: 10.1002/cjoc.v39.6 |
|
(b) Yao, R.-C.; Ling, Y.-J.; Chen, W.-B.; Lu, L.; Shen, Q. Org. Process Res. Dev. 2022, 26, 299.
doi: 10.1021/acs.oprd.1c00381 |
|
| [17] |
(a) Ge, H.-M.; Shen, Q. Org. Chem. Front. 2019, 10, 2205.
|
|
(b) Ge, H.-M.; Wu, B.-T.; Liu, Y.-F.; Wang, H.-Y.; Shen, Q. ACS Catal. 2020, 10, 12414.
doi: 10.1021/acscatal.0c03776 |
|
|
(c) Yao, R.-C.; Chen, W.-B.; Shen, Q. Chin. J. Org. Chem. 2021, 41, 2684. (in Chinese)
doi: 10.6023/cjoc202103004 |
|
|
(姚瑞超, 陈文博, 沈其龙, 有机化学, 2021, 41, 2684.)
doi: 10.6023/cjoc202103004 |
|
| [18] |
Cheng, Y. Z.; Yuan, X. G.; Ma, J.; Yu, S. Y. Chem.-Eur. J. 2015, 21, 8355.
doi: 10.1002/chem.v21.23 |
| [1] | Wenwen Chen, Qin Zhang, Songyue Zhang, Fangfang Huang, Xinyin Zhang, Jianfeng Jia. Visible Light Promoted Coupling Reaction of Alkynyl Iodide and Sodium Sulphinate without Photocatalyst [J]. Chinese Journal of Organic Chemistry, 2024, 44(2): 584-592. |
| [2] | Yanshuo Zhu, Hongyan Wang, Penghua Shu, Ke'na Zhang, Qilin Wang. Recent Advances on Alkoxy Radicals-Mediated C(sp3)—H Bond Functionalization via 1,5-Hydrogen Atom Transfer [J]. Chinese Journal of Organic Chemistry, 2024, 44(1): 1-17. |
| [3] | Si Wen, Yuhao Ding, Qingyu Tian, Jin Ge, Guolin Cheng. Rhodium(III)-Catalyzed Synthesis of CF3-1H-benzo[de][1,8]naph-thyridines via C—H Activation/Annulation of Benzimidates and CF3-Imidoyl Sulfoxonium Ylides [J]. Chinese Journal of Organic Chemistry, 2024, 44(1): 291-300. |
| [4] | Dandan Sui, Nannan Cen, Ruoqu Gong, Yang Chen, Wenbo Chen. Supporting-Electrolyte-Free Electrochemical Synthesis of Trifluoromethylated Oxindoles in Continuous Flow [J]. Chinese Journal of Organic Chemistry, 2023, 43(9): 3239-3245. |
| [5] | Sijie Fan, Wuheng Dong, Caiyun Liang, Guichao Wang, Yao Yuan, Zuodong Yin, Zhaoguo Zhang. Visible Light-Induced Radical Cyclization for the Construction of 4-Aryl-1,2-dihydronaphthalenes [J]. Chinese Journal of Organic Chemistry, 2023, 43(9): 3277-3286. |
| [6] | Hu Ma, Danfeng Huang, Kehu Wang, Duoduo Tang, Yang Feng, Yuanyuan Reng, Junjiao Wang, Yulai Hu. Synthesis of 3-Trifluoromethylpyrazole Derivatives [J]. Chinese Journal of Organic Chemistry, 2023, 43(9): 3257-3267. |
| [7] | Wei Xu, Hongbin Zhai, Bin Cheng, Taimin Wang. Visible Light-Induced Pd-Catalyzed Heck Reactions [J]. Chinese Journal of Organic Chemistry, 2023, 43(9): 3035-3054. |
| [8] | Xiaona Yang, Hongyu Guo, Rong Zhou. Progress in Visible-Light Promoted Transformations of Organosilicon Compounds [J]. Chinese Journal of Organic Chemistry, 2023, 43(8): 2720-2742. |
| [9] | Yanhua Gao, Yinpan Zhang, Yan Zhang, Tao Song, Yong Yang. Visible-Light-Induced Aerobic Oxidation of Alcohols over Surface Oxygen Vacancies-Enriched Nb2O5 [J]. Chinese Journal of Organic Chemistry, 2023, 43(7): 2572-2579. |
| [10] | Rui Wang, Lang Gao, Cen Zhou, Xiao Zhang. Haloperfluoroalkylation of Unactivated Terminal Alkenes over Phenylphenothiazine-Based Porous Organic Polymers [J]. Chinese Journal of Organic Chemistry, 2023, 43(3): 1136-1145. |
| [11] | Jinxiao Zhao, Tonghui Wei, Sen Ke, Yi Li. Visible Light-Catalyzed Synthesis of Difluoroalkylated Polycyclic Indoles [J]. Chinese Journal of Organic Chemistry, 2023, 43(3): 1102-1114. |
| [12] | Shuyong Song, Senmiao Xu. Recent Progress in Selective C-F Bond Activation of Trifluoromethyl Alkenes [J]. Chinese Journal of Organic Chemistry, 2023, 43(2): 411-425. |
| [13] | Yasir Mumtaz, Jie Liu, Xin Huang. Copper-Promoted Trifluoromethylthiolation of Anilines with CF3SO2Na [J]. Chinese Journal of Organic Chemistry, 2023, 43(2): 679-685. |
| [14] | Xiang Chen, Wen-Tao Ouyang, Xiao Li, Wei-Min He. Visible-Light Induced Organophotocatalysis for the Synthesis of Difluoroethylated Benzoxazines [J]. Chinese Journal of Organic Chemistry, 2023, 43(12): 4213-4219. |
| [15] | Jiajie Zhu, Yi Wan, Qiyang Yuan, Jinlian Wei, Yongqiang Zhang. Research of Visible Light/Lewis Base Dual Catalytic Defluorinative Silylation of Trifluoromethyl-Substituted Alkenes [J]. Chinese Journal of Organic Chemistry, 2023, 43(10): 3623-3634. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||