Chinese Journal of Organic Chemistry ›› 2024, Vol. 44 ›› Issue (10): 3091-3105.DOI: 10.6023/cjoc202406044 Previous Articles Next Articles
Special Issue: 二氧化碳专题合集
REVIEWS
许立锋a, 武安国a, 于芳羽a, 李红茹a,b,*( ), 何良年a,*(
), 何良年a,*( )
)
收稿日期:2024-06-28
修回日期:2024-07-24
发布日期:2024-08-30
基金资助:
Lifeng Xua, Anguo Wua, Fangyu Yua, Hongru Lia,b,*( ), Liangnian Hea,*(
), Liangnian Hea,*( )
)
Received:2024-06-28
Revised:2024-07-24
Published:2024-08-30
Contact:
*E-mail: Supported by:Share
Lifeng Xu, Anguo Wu, Fangyu Yu, Hongru Li, Liangnian He. Progress on Renewable Energy-Driven Synthesis of Cyclic Carbonates from CO2[J]. Chinese Journal of Organic Chemistry, 2024, 44(10): 3091-3105.
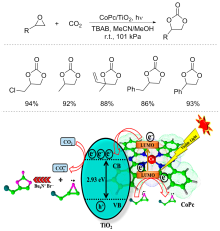


| Entry | Epoxide (n/mmol) | Catalyst (dosage) | TBAB/mol% | Solvente (V/mL) | Reaction condition | Yield/% | Ref. |
|---|---|---|---|---|---|---|---|
| 1 | Propylene oxide (1) | CoPc/TiO2 (100 mg) | 10 | S1/S2 (10/5) | 24 h, 20 W LED | 92 | [ |
| 2 | Propylene oxide (3) | Ti18Bi4O29Bz26 (20 μmol) | 1.6 | 14 h, 300 W Xe lamp | >99 | [ | |
| 3b | Propylene oxide (1.4) | BiNbO4/r-GO (50 mg) | 2 | S1/S2 (10/2) | 24 h, 300 W Hg lamp | 65 | [ |
| 4c | Propylene oxide (1.4) | FeNbO4/r-GO (50 mg) | 2 | S1/S2 (4/1) | 24 h, 500 W Hg lamp | 57 | [ |
| 5 | Phenyl oxirane (44) | W18O49/g-C3N4 (30 mg) | 2.5 | 4 h, 300 W Xe lamp | 60 | [ | |
| 6 | Propylene oxide (71) | g-C3N4/Ag (10 mg) | 0.7 | 6 h, 300 W Xe lamp | [ | ||
| 7 | Phenyl oxirane (0.15) | Pd/SCNT-500 (10 mg) | 67 | 24 h, 0.15w/cm2 455nm | 97 | [ | |
| 8d | Propylene oxide (10) | Zr-Thia/g-CN (50 mg) | 2.5 | 30 h, 250w Hg lamp | 90.8 | [ | |
| 9c | Propylene oxide (1.4) | BiNbO4/NH2-MIL-125(Ti) (50 mg) | 2 | S1/S2 (5/1) | 72 h, 300 W Hg lamp | 74 | [ |
| 10c | Propylene oxide (1.4) | FeNbO4/NH2-MIL-125(Ti) (50 mg) | 2 | S1/S2 (4/1) | 72 h, 300 W Hg lamp | 52 | [ |
| 11 | Propylene oxide (4.5) | Bi-PCN-224 (30 mg) | 11 | 6 h, 300 W Xe lamp | 99 | [ | |
| 12 | Propylene oxide (0.1) | UiO-bpydc (Zn) (20 mg) | 500 | S3 (3) | 9 h, 300 W Xe lamp | 90 | [ |
| 13 | Epichlorohydrin (20) | PCN-224 (Mg) (0.5 mmol) | 1 | 6 h, LED light (3*30 W) | 99 | [ | |
| 14 | Epichlorohydrin (38) | Fe-BDC (15 mg) | 0.8 | 4 h, 300 W Xe lamp | 45 | [ | |
| 15 | Epichlorohydrin (12.75) | Fe-DBP (10 mg) | 8 | 12 h, 1000 W Xe lamp. | 97 | [ | |
| 16 | Epichlorohydrin (0.2) | TpPa-1 (5 mg) | 5 | S1 (5) | 8 h, Blue light 455nm | 82 | [ |
| 17 | Propylene oxide (0.25) | OH-P [5]-on-COF (1.5 mg) | 5 | 8 h, LED lamp | 99 | [ | |
| 18 | Epichlorohydrin (1) | 9,10-Anthraquinone (10 mol%) | 10 | S1 (10) | 12 h, 20 W LED | 93 | [ |
| Entry | Epoxide (n/mmol) | Catalyst (dosage) | TBAB/mol% | Solvente (V/mL) | Reaction condition | Yield/% | Ref. |
|---|---|---|---|---|---|---|---|
| 1 | Propylene oxide (1) | CoPc/TiO2 (100 mg) | 10 | S1/S2 (10/5) | 24 h, 20 W LED | 92 | [ |
| 2 | Propylene oxide (3) | Ti18Bi4O29Bz26 (20 μmol) | 1.6 | 14 h, 300 W Xe lamp | >99 | [ | |
| 3b | Propylene oxide (1.4) | BiNbO4/r-GO (50 mg) | 2 | S1/S2 (10/2) | 24 h, 300 W Hg lamp | 65 | [ |
| 4c | Propylene oxide (1.4) | FeNbO4/r-GO (50 mg) | 2 | S1/S2 (4/1) | 24 h, 500 W Hg lamp | 57 | [ |
| 5 | Phenyl oxirane (44) | W18O49/g-C3N4 (30 mg) | 2.5 | 4 h, 300 W Xe lamp | 60 | [ | |
| 6 | Propylene oxide (71) | g-C3N4/Ag (10 mg) | 0.7 | 6 h, 300 W Xe lamp | [ | ||
| 7 | Phenyl oxirane (0.15) | Pd/SCNT-500 (10 mg) | 67 | 24 h, 0.15w/cm2 455nm | 97 | [ | |
| 8d | Propylene oxide (10) | Zr-Thia/g-CN (50 mg) | 2.5 | 30 h, 250w Hg lamp | 90.8 | [ | |
| 9c | Propylene oxide (1.4) | BiNbO4/NH2-MIL-125(Ti) (50 mg) | 2 | S1/S2 (5/1) | 72 h, 300 W Hg lamp | 74 | [ |
| 10c | Propylene oxide (1.4) | FeNbO4/NH2-MIL-125(Ti) (50 mg) | 2 | S1/S2 (4/1) | 72 h, 300 W Hg lamp | 52 | [ |
| 11 | Propylene oxide (4.5) | Bi-PCN-224 (30 mg) | 11 | 6 h, 300 W Xe lamp | 99 | [ | |
| 12 | Propylene oxide (0.1) | UiO-bpydc (Zn) (20 mg) | 500 | S3 (3) | 9 h, 300 W Xe lamp | 90 | [ |
| 13 | Epichlorohydrin (20) | PCN-224 (Mg) (0.5 mmol) | 1 | 6 h, LED light (3*30 W) | 99 | [ | |
| 14 | Epichlorohydrin (38) | Fe-BDC (15 mg) | 0.8 | 4 h, 300 W Xe lamp | 45 | [ | |
| 15 | Epichlorohydrin (12.75) | Fe-DBP (10 mg) | 8 | 12 h, 1000 W Xe lamp. | 97 | [ | |
| 16 | Epichlorohydrin (0.2) | TpPa-1 (5 mg) | 5 | S1 (5) | 8 h, Blue light 455nm | 82 | [ |
| 17 | Propylene oxide (0.25) | OH-P [5]-on-COF (1.5 mg) | 5 | 8 h, LED lamp | 99 | [ | |
| 18 | Epichlorohydrin (1) | 9,10-Anthraquinone (10 mol%) | 10 | S1 (10) | 12 h, 20 W LED | 93 | [ |
| Entry | Catalystb | Zn form | Reaction condition | Light intensitya | Product | Yield/% | Ref. |
|---|---|---|---|---|---|---|---|
| 1 | HPC-800 | Zn atom | 100 kPa CO2, 10 h | 300 mW•cm–2 | 4-Bromomethyl-1,3-dioxolan-2-one | 94 | [ |
| 2 | Zn SA-NC | Zn atom | 100 kPa CO2, 16 h | 300 mW•cm–2 | 4-Chloromethyl-1,3-dioxolan-2-one | 99 | [ |
| 3 | ZNC-800 | Zn atom | 100 kPa CO2, 10 h | 1000 mW•cm–2 | 4-Methyl-1,3-dioxolan-2-one | 94 | [ |
| 4 | Zn-Asp-300 | ZnO | 100 kPa CO2, 4 h | 152.5 mW•cm–2 | 4-Chloromethyl-1,3-dioxolan-2-one | 92 | [ |
| 5 | ZNPC-600 | ZnO | 100 kPa CO2, 6 h | 500 mW•cm–2 | 4-Chloromethyl-1,3-dioxolan-2-one | 99 | [ |
| 6 | ZnS / NPC-2 | ZnS | 100 kPa CO2, 12 h | 300 mW•cm–2 | 4-Chloromethyl-1,3-dioxolan-2-one | 98 | [ |
| 7 | ZnO/NC-L | ZnO | 100 kPa CO2, 6 h | 120 mW•cm–2 | 4-Chloromethyl-1,3-dioxolan-2-one | 76 | [ |
| Entry | Catalystb | Zn form | Reaction condition | Light intensitya | Product | Yield/% | Ref. |
|---|---|---|---|---|---|---|---|
| 1 | HPC-800 | Zn atom | 100 kPa CO2, 10 h | 300 mW•cm–2 | 4-Bromomethyl-1,3-dioxolan-2-one | 94 | [ |
| 2 | Zn SA-NC | Zn atom | 100 kPa CO2, 16 h | 300 mW•cm–2 | 4-Chloromethyl-1,3-dioxolan-2-one | 99 | [ |
| 3 | ZNC-800 | Zn atom | 100 kPa CO2, 10 h | 1000 mW•cm–2 | 4-Methyl-1,3-dioxolan-2-one | 94 | [ |
| 4 | Zn-Asp-300 | ZnO | 100 kPa CO2, 4 h | 152.5 mW•cm–2 | 4-Chloromethyl-1,3-dioxolan-2-one | 92 | [ |
| 5 | ZNPC-600 | ZnO | 100 kPa CO2, 6 h | 500 mW•cm–2 | 4-Chloromethyl-1,3-dioxolan-2-one | 99 | [ |
| 6 | ZnS / NPC-2 | ZnS | 100 kPa CO2, 12 h | 300 mW•cm–2 | 4-Chloromethyl-1,3-dioxolan-2-one | 98 | [ |
| 7 | ZnO/NC-L | ZnO | 100 kPa CO2, 6 h | 120 mW•cm–2 | 4-Chloromethyl-1,3-dioxolan-2-one | 76 | [ |
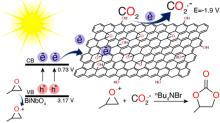
| Entry | Cathode|anode | Supporting electrolyte | Solvent | Reaction condition | Epoxide | Yield/% | Ref. |
|---|---|---|---|---|---|---|---|
| 1 | Ss|Mg | KBr, Ni(cyclam)Br2 | DMF | r.t., 100 kPa | Styrene oxide | 92 | [ |
| 2 | Cu|Mg/Al | [BMlm][BF4] | r.t., 100 kPa | Propylene oxide | 92 | [ | |
| 3 | CuNPs|Mg | 0.1 mol/L TEAI | MeCN | 25 ℃, 100 kPa | Propylene oxide | 86 | [ |
| 4 | Cu/CS|Mg | 0.1 mol/L TEAI | MeCN | r.t., 100 kPa | Propylene oxide | 94.7 | [ |
| 5 | AgNPs|Mg | 0.1 mol/L TEAI | MeCN | 25 ℃, 100 kPa | Propylene oxide | 70 | [ |
| 6 | Ss|Mg | TBAI | MeCN | r.t., 100 kPa | (R)-Styrene oxide | 59 (91% ee) | [ |
| 7 | C|Pt | TBAP, Ni(Ⅱ) complex | MeCN | r.t., 100 kPa | Styrene oxide | 100 | [ |
| 8 | Cu(ND)/CP|Pt | ZnCl2, TBAB | MeCN | r.t., 100 kPa | Styrene oxide | 74.9 | [ |
| 9 | HNSs|Pt | ZnCl2, TBAB | MeCN | r.t., 100 kPa | Styrene oxide | 66.9 | [ |
| 10 | Ti/TiO2-CNT-Pt|C | [APMIm]DCA | MeCN | 50 ℃, 100 kPa | Styrene oxide | 95 | [ |
| Entry | Cathode|anode | Supporting electrolyte | Solvent | Reaction condition | Epoxide | Yield/% | Ref. |
|---|---|---|---|---|---|---|---|
| 1 | Ss|Mg | KBr, Ni(cyclam)Br2 | DMF | r.t., 100 kPa | Styrene oxide | 92 | [ |
| 2 | Cu|Mg/Al | [BMlm][BF4] | r.t., 100 kPa | Propylene oxide | 92 | [ | |
| 3 | CuNPs|Mg | 0.1 mol/L TEAI | MeCN | 25 ℃, 100 kPa | Propylene oxide | 86 | [ |
| 4 | Cu/CS|Mg | 0.1 mol/L TEAI | MeCN | r.t., 100 kPa | Propylene oxide | 94.7 | [ |
| 5 | AgNPs|Mg | 0.1 mol/L TEAI | MeCN | 25 ℃, 100 kPa | Propylene oxide | 70 | [ |
| 6 | Ss|Mg | TBAI | MeCN | r.t., 100 kPa | (R)-Styrene oxide | 59 (91% ee) | [ |
| 7 | C|Pt | TBAP, Ni(Ⅱ) complex | MeCN | r.t., 100 kPa | Styrene oxide | 100 | [ |
| 8 | Cu(ND)/CP|Pt | ZnCl2, TBAB | MeCN | r.t., 100 kPa | Styrene oxide | 74.9 | [ |
| 9 | HNSs|Pt | ZnCl2, TBAB | MeCN | r.t., 100 kPa | Styrene oxide | 66.9 | [ |
| 10 | Ti/TiO2-CNT-Pt|C | [APMIm]DCA | MeCN | 50 ℃, 100 kPa | Styrene oxide | 95 | [ |
| [1] |
(a) Yu, B.; He, L. N. ChemSusChem 2014, 8, 52.
|
|
(b) Sakakura, T.; Choi, J.-C.; Yasuda, H. Chem. Rev. 2007, 107, 2365.
|
|
|
(c) Qiu, L. Q.; Yao, X. Y.; Zhang, Y. K.; Li, H. R.; He, L. N. J. Org. Chem. 2023, 88, 4942.
|
|
|
(d) Qiu, L. Q.; Li, H. R.; He, L. N. Acc. Chem. Res. 2023, 56, 2225.
|
|
| [2] |
Lang, X. D.; He, L. N. Chem. Rec. 2016, 16, 1337.
|
| [3] |
Prajapati, P. K.; Kumar, A.; Jain, S. L. ACS Sustainable Chem. Eng. 2018, 6, 7799.
|
| [4] |
Liu, C.; Niu, H.; Wang, D.; Gao, C.; Said, A.; Liu, Y.; Wang, G.; Tung, C.-H.; Wang, Y. ACS Catal. 2022, 12, 8202.
|
| [5] |
(a) Ahmed, S. H.; Bakiro, M.; Alzamly, A. Materialia 2020, 12, 100781.
|
|
(b) Bakiro, M.; Hussein Ahmed, S.; Alzamly, A. ACS Sustainable Chem. Eng. 2020, 8, 12072.
|
|
| [6] |
Cheng, R.; Wang, A.; Sang, S.; Liang, H.; Liu, S.; Tsiakaras, P. Chem. Eng. J. 2023, 466, 142982.
|
| [7] |
Gong, X.; Zhang, Y.; Xu, Y.; Zhai, G.; Liu, X.; Bao, X.; Wang, Z.; Liu, Y.; Wang, P.; Cheng, H.; Fan, Y.; Dai, Y.; Zheng, Z.; Huang, B. ACS Appl. Mater. Interfaces 2022, 14, 51029.
|
| [8] |
Jiang, H.; Zang, C.; Guo, L.; Gao, X. Sci. Total Environ. 2022, 838, 155920.
|
| [9] |
Kumar, A.; Samanta, S.; Srivastava, R. ACS Appl. Nano Mater. 2021, 4, 6805.
|
| [10] |
(a) Hussein Ahmed, S.; Bakiro, M.; Alzamly, A. Molecules 2021, 26, 1693.
|
|
(b) Bakiro, M.; Ahmed, S. H.; Alzamly, A. J. Environ. Chem. Eng. 2020, 8, 104461.
|
|
| [11] |
Zhai, G.; Liu, Y.; Lei, L.; Wang, J.; Wang, Z.; Zheng, Z.; Wang, P.; Cheng, H.; Dai, Y.; Huang, B. ACS Catal. 2021, 11, 1988.
|
| [12] |
Zhai, G.; Liu, Y.; Mao, Y.; Zhang, H.; Lin, L.; Li, Y.; Wang, Z.; Cheng, H.; Wang, P.; Zheng, Z.; Dai, Y.; Huang, B. Appl. Catal., B 2022, 301, 120793.
|
| [13] |
Das, R.; Manna, S. S.; Pathak, B.; Nagaraja, C. M. ACS Appl. Mater. Interfaces 2022, 14, 33285.
|
| [14] |
Zhang, H.; Si, S.; Zhai, G.; Li, Y.; Liu, Y.; Cheng, H.; Wang, Z.; Wang, P.; Zheng, Z.; Dai, Y.; Liu, T. X.; Huang, B. Appl. Catal., B 2023, 337, 122909.
|
| [15] |
Shi, Q.; Chen, M.-H.; Xiong, J.; Li, T.; Feng, Y.-Q.; Zhang, B. Chem. Eng. J. 2024, 481, 148301.
|
| [16] |
Das, A.; Mondal, R. K.; Chakrabortty, P.; Riyajuddin, S.; Chowdhury, A. H.; Ghosh, S.; Khan, A.; Ghosh, K.; Islam, S. M. Mol. Catal. 2021, 499, 111253.
|
| [17] |
Li, X.; Niu, X.; Fu, P.; Song, Y.; Zhang, E.; Dang, Y.; Yan, J.; Feng, G.; Lei, S.; Hu, W. Appl. Catal., B 2024, 350, 123943.
|
| [18] |
Saini, S.; Khan, S. R.; Gour, N. K.; Chandra Deka, R.; Jain, S. L. Green Chem. 2022, 24, 3644.
|
| [19] |
Yang, Q.; Yang, C. C.; Lin, C. H.; Jiang, H. L. Angew. Chem. Int. Ed. 2019, 58, 3511.
|
| [20] |
Gong, L.; Sun, J.; Liu, Y.; Yang, G. J. Mater. Chem. A 2021, 9, 21689.
|
| [21] |
Duan, C.; Ding, M.; Feng, Y.; Cao, M.; Yao, J. Sep. Purif. Technol. 2022, 285, 120359.
|
| [22] |
Liu, Y.; Chen, Y.; Liu, Y.; Chen, Z.; Yang, H.; Yue, Z.; Fang, Q.; Zhi, Y.; Shan, S. J. Catal. 2022, 407, 65.
|
| [23] |
Dai, W.; Zou, M.; Long, J.; Li, B.; Zhang, S.; Yang, L.; Wang, D.; Mao, P.; Luo, S.; Luo, X. Appl. Surf. Sci. 2021, 540, 148311.
|
| [24] |
Rong, W.; Ding, M.; Ma, P.; Kong, S.; Yao, J. J. Ind. Eng. Chem. 2024, 129, 682.
|
| [25] |
Tang, F.; Wang, L.; Ma, L.; Fang, Y.; Huang, J.; Liu, Y.-N. J. CO2 Util. 2021, 45, 101431.
|
| [26] |
Yang, Q.; Peng, H.; Zhang, Q.; Qian, X.; Chen, X.; Tang, X.; Dai, S.; Zhao, J.; Jiang, K.; Yang, Q.; Sun, J.; Zhang, L.; Zhang, N.; Gao, H.; Lu, Z.; Chen, L. Adv. Mater. 2021, 33, 2103186
|
| [27] |
Wang, Y.; Liu, H.; Shi, Q.; Miao, Z.; Duan, H.; Wang, Y.; Rong, H.; Zhang, J. Angew. Chem. Int. Ed. 2024, e202404911.
|
| [28] |
Paliwal, K. S.; Sarkar, D.; Mitra, A.; Mahalingam, V. ChemPlus-Chem 2023, 88, e202300448.
|
| [29] |
Wang, T.; Chen, F.; Jiang, L.; Li, J.; Chen, K.; Gao, J. Inorg. Chem. 2024, 63, 4224.
|
| [30] |
Guo, Q.; Xia, S.-G.; Li, X.-B.; Wang, Y.; Liang, F.; Lin, Z.-S.; Tung, C.-H.; Wu, L.-Z. Chem. Commun. 2020, 56, 7849.
|
| [31] |
Yang, Z.; Xie, Y.; Feng, Y.; Yao, J. J. Environ. Chem. Eng. 2024, 12, 112310.
|
| [32] |
(a) Chen, H.; Fan, L.; Zhang, X. ACS Appl. Mater. Interfaces 2020, 12, 54884.
|
|
(b) Yin, M.; Wang, L.; Tang, S. ACS Catal. 2023, 13, 13021.
|
|
|
(c) Ding, M.; Jiang, H.-L. ACS Catal. 2018, 8, 3194.
|
|
| [33] |
Sharma, N.; Dhankhar, S. S.; Nagaraja, C. M. Microporous Mesoporous Mater. 2019, 280, 372.
|
| [34] |
Zhou, X.; Zhang, H.; Cheng, H.; Wang, Z.; Wang, P.; Zheng, Z.; Dai, Y.; Xing, D.; Liu, Y.; Huang, B. J. Colloid Interface Sci. 2024, 658, 805.
|
| [35] |
Fang, Z.; Deng, Z.; Wan, X.; Li, Z.; Ma, X.; Hussain, S.; Ye, Z.; Peng, X. ppl. Catal., B 2021, 296, 120329.
|
| [36] |
Duan, C.; Xie, Y.; Ding, M.; Feng, Y.; Yao, J. J. CO2 Util. 2022, 64, 102158.
|
| [37] |
Feng, Y.; Cao, Y.; Zhu, J.; Han, H.; Liu, Y.; Li, X.; Zhao, S.; Yang, J.; Fang, Z.; He, W.; Yang, Z.; Guo, K. J. Cleaner Prod. 2024, 440, 141002.
|
| [38] |
Ding, L.-G.; Yao, B.-J.; Wu, W.-X.; Yu, Z.-G.; Wang, X.-Y.; Kan, J.-L.; Dong, Y.-B. Inorg. Chem. 2021, 60, 12591.
|
| [39] |
Zhang, L.; Tu, X.; Chen, Y.; Han, W.; Chen, L.; Sun, C.; Zhu, S.; Song, Y.; Zheng, H. Mol. Catal. 2023, 538, 112971.
|
| [40] |
(a) Wu, Y.; Yu, X.-F.; Du, Y.; Xia, L.; Guo, Q.; Zhang, K.; Zhang, W.; Liu, S.; Peng, Y.; Li, Z.; Yang, X. Appl. Catal., B 2023, 331, 122732.
|
|
(b) Zhang, W.; Li, Z.; Yu, X.-F.; Zhang, K.; Liu, S.; Du, Y.; Guo, Q.; Zhang, L.; Ding, X.; Tang, H.; Peng, Y.; Yang, X. Inorg. Chem. 2024, 63, 2954.
|
|
| [41] |
Tascedda, P.; Weidmann, M.; Dinjus, E.; Duach, E. Appl. Organomet. Chem. 2001, 15, 141.
|
| [42] |
Yang, H.; Gu, Y.; Deng, Y.; Shi, F. Chem. Commun. 2002, 274.
|
| [43] |
Wu, L.-X; Yang, H.-P; Wang, H.; Lu, J.-X. RSC Adv. 2015, 5, 23189.
|
| [44] |
Zhang, J.-J.; Shan, S.-L.; Shi, Y.; Hou, Y.; Wang, H.; Lu, J.-X. J. Electroanal. Chem. 2021, 882, 114962.
|
| [45] |
Wu, L.-X.; Yang, H.-P.; Guan, Y.-B.; Yang, M.-P.; Wang, H.; Lu, J.-X. Int. J. Electrochem. Sci. 2017, 12, 8963.
|
| [46] |
Xiao, Y.; Chen, B.-L.; Yang, H.-P.; Wang, H.; Lu, J.-X. Electrochem. Commun. 2014, 43, 71.
|
| [47] |
Khoshro, H.; Zare, H. R.; Namazian, M.; Jafari, A. A.; Gorji, A. Electrochim. Acta 2013, 113, 263.
|
| [48] |
Li, W.-Z; Qi, K.; Lu, X.-Y; Qi, Y.; Zhang, J.-L; Zhang, B.-S; Qi, W. Chem.-Eur. J. 2022, 28, e202200622
|
| [49] |
Dai, X.-Y; Qi, K.; Liu, C.-W; Lu, X.-Y; Qi, W. Carbon 2023, 202, 51.
|
| [50] |
Hu, Y. -L.; Liu, X. -B.; Rong, Q. Green Chem. Lett. Rev. 2023, 16, 2163192.
|
| [51] |
Gao, X.-F; Yuan, G.-Q; Chen, H.-J; Jiang, H.-F; Li, Y.-W; Qi, C-R. Electrochem. Commun. 2013, 34, 242.
|
| [52] |
Wang, H; Wu, L.-X; Lan, Y.-C; Zhao, J.-Q; Lu, J.-X. Int. J. Electrochem. Sci. 2011, 6, 4218.
|
| [53] |
Wu, L. -X; Wang, H; Tu, J -Z.-Y; Ding, B. -B; Xiao, Y; Lu, J.-X. Int. J. Electrochem. Sci. 2012, 7, 11540.
|
| [54] |
Wang, H.; Wu, L.-X.; Zhao, J.-Q.; Li, R.-N.; Zhang, A.-J.; Kajiura, H.; Li, Y.-M.; Lu, J.-X. Greenhouse Gas. Sci. Technol. 2012, 2, 59.
|
| [55] |
(a) Liu, J.; Yang, G.; Liu, Y.; Wu, D.; Hu, X.; Zhang, Z. Green Chem. 2019, 21, 3834.
|
|
(b) Iglesias, D.; Tinajero, C.; Marchetti, S.; Roppolo, I.; Zanatta, M.; Sans, V. Green Chem. 2023, 25, 9934.
|
|
| [56] |
(a) Chung, M.; Maalouf, J. H.; Adams, J. S.; Jiang, C.; Román-Leshkov, Y.; Manthiram, K. Science 2024, 383, 49.
pmid: 32527828 |
|
(b) Leow, W. R.; Lum, Y.; Ozden, A.; Wang, Y.; Nam, D.-H.; Chen, B.; Wicks, J.; Zhuang, T.-T.; Li, F.; Sinton, D.; Sargent, E. H. Science 2020, 368, 1228.
doi: 10.1126/science.aaz8459 pmid: 32527828 |
|
|
(c) Tao, Y.; Huang, C.; Lu, Q. Nat. Catal. 2023, 6, 1107.
pmid: 32527828 |
|
|
(d) Yang, Y.; Yuan, X.; Wang, Q.; Wan, S.; Lin, C.; Lu, S.; Zhong, Q.; Zhang, K. Angew. Chem. Int. Ed. 2024, 63, e202314383.
pmid: 32527828 |
|
|
(e) Seitz, A. K.; Kohlpaintner, P. J.; van Lingen, T.; Dyga, M.; Sprang, F.; Zirbes, M.; Waldvogel, S. R.; Gooßen, L. J. Angew. Chem. Int. Ed. 2022, 61, e202117563.
pmid: 32527828 |
|
| [57] |
Zhang, J.-J.; Li, S.-M.; Shi, Y.; Hu, Q.-L.; Wang, H.; Lu, J.-X. New J. Chem. 2020, 44, 11817.
|
| [1] | Dongsen Duan, Yuan Ma, Yubo Liu, Fu Cheng, Daoyong Zhu, Shaohua Wang. Visible Light-Induced Decarbon-Carboxylation of Activated Alkenes by Carbon Dioxide [J]. Chinese Journal of Organic Chemistry, 2024, 44(5): 1675-1685. |
| [2] | Xiaolin Jiang, Chaoyang Wang, Liyuan Wu, Yuehui Li. Advances in Catalytic Conversion of CO2 with Carbazole-Based Molecules and Polymers [J]. Chinese Journal of Organic Chemistry, 2024, 44(5): 1423-1444. |
| [3] | Kun Xia, Kaifa Zhang, Sher Wali Khan, Abdukader Ablimit. Advances in Three-Component Coupling Reactions Involving CO2 [J]. Chinese Journal of Organic Chemistry, 2024, 44(5): 1506-1525. |
| [4] | Shuai Lv, Gangguo Zhu, Jinzhong Yao, Hongwei Zhou. Research Progress in Preparation of Carboxylic Acids by Electrochemical Mediated Oxidative Carboxylation and Reductive Carboxylation of Carbon Dioxide [J]. Chinese Journal of Organic Chemistry, 2024, 44(3): 780-808. |
| [5] | Jianwen Li, Tao Wang, Sheng Tao, Fei Chen, Min Li, Ning Liu. N-Heterocyclic Carbene-Pyridine Molybdenum Complex Supported over SBA-15 for Converting of Carbon Dioxide into Cyclic Carbonates [J]. Chinese Journal of Organic Chemistry, 2024, 44(10): 3213-3222. |
| [6] | Yuyuan Zhang, Changjie Yang, Haitao Tang, Yingming Pan. Heterogeneous Catalytic Fixation of Carbon Dioxide for Synthesis of Carbonyl Derivatives [J]. Chinese Journal of Organic Chemistry, 2024, 44(10): 3077-3090. |
| [7] | Yanwei Wang, Weiwei Chen, Dan Yuan, Yong Zhang, Yingming Yao. Advances in the Reactions of CO2 and Epoxides Catalyzed by Heterometallic Complexes [J]. Chinese Journal of Organic Chemistry, 2024, 44(10): 3063-3076. |
| [8] | Xiaotong Gao, Yuqing Zhong, Nan Feng, Ying Sun, Deyong Yang, Feng Zhou. Recent Advances in Electrochemical Carboxylation of Inert Chemical Bonds with Carbon Dioxide [J]. Chinese Journal of Organic Chemistry, 2024, 44(10): 3043-3062. |
| [9] | Panfeng Yuan, Canming Zhu, Qingyuan Meng. Advances in the Synthesis of Carboxylic Acid by Photochemical Conversion of CO2 [J]. Chinese Journal of Organic Chemistry, 2024, 44(10): 2997-3042. |
| [10] | Wenke Li, Beiqi Sun, Lei Zhang, Fanyang Mo. Recent Advances in Photocatalytic Carboxylation Based on Free Radical Process [J]. Chinese Journal of Organic Chemistry, 2024, 44(10): 2961-2996. |
| [11] | Jing Hou, Yan Huang, Hao Li, Yuancui Wan, Yu Shao, Lewu Zhan, Dinghai Wang, Bindong Li. Recent Advances in the Application of Carbon Dioxide Radical Anion [J]. Chinese Journal of Organic Chemistry, 2024, 44(10): 3117-3135. |
| [12] | Jiayuan Li, Yaping Yi, Chanjuan Xi. Advances in Dearomative Carboxylation of Aromatic Compounds with Carbon Dioxide [J]. Chinese Journal of Organic Chemistry, 2024, 44(10): 3136-3146. |
| [13] | Aolong Zhang, Han Yang, Peidong Cheng, Yang Yao, Song Sun. Visible-Light Photoredox-Catalyzed Carbon/Carboxylation of Alkenes with Malonates and CO2 [J]. Chinese Journal of Organic Chemistry, 2024, 44(10): 3159-3168. |
| [14] | Xu Liao, Zeyu Wang, Wufei Tang, Jinqing Lin. Progress in Porous Organic Polymer for Chemical Fixation of Carnbon Dioxide [J]. Chinese Journal of Organic Chemistry, 2023, 43(8): 2699-2710. |
| [15] | Zijie Song, Jun Liu, Ying Bai, Jiayun Li, Jiajian Peng. Progress in Catalysis Transformation of Carbon Dioxide through Hydrosilylation [J]. Chinese Journal of Organic Chemistry, 2023, 43(6): 2068-2080. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||