Chinese Journal of Organic Chemistry ›› 2025, Vol. 45 ›› Issue (7): 2486-2500.DOI: 10.6023/cjoc202411022 Previous Articles Next Articles
ARTICLES
卜淑甜a, 王永a, 殷文剑a, 温青云a, 王佩c, 朱伟明a,b,*( )
)
收稿日期:2024-11-27
修回日期:2025-01-11
发布日期:2025-02-14
基金资助:
Shutian Bua, Yong Wanga, Wenjian Yina, Qingyun Wena, Pei Wangc, Weiming Zhua,b,*( )
)
Received:2024-11-27
Revised:2025-01-11
Published:2025-02-14
Contact:
*E-mail: weimingzhu@ouc.edu.cn
Supported by:Share
Shutian Bu, Yong Wang, Wenjian Yin, Qingyun Wen, Pei Wang, Weiming Zhu. Staurosporine Natural Products Derived from Marine Streptomyces sp. OUCMDZ-5522[J]. Chinese Journal of Organic Chemistry, 2025, 45(7): 2486-2500.
| Position | δC (type) | δH (mult. J in Hz) | COSY | HMBC | NOESY |
|---|---|---|---|---|---|
| 1 | 109.9 (CH) | 7.75 (d, 8.2) | 2 | 3, 4a | |
| 2 | 127.2 (CH) | 7.61 (t, 7.4) | 1, 3 | 4, 13a | |
| 3 | 120.7 (CH) | 7.43 (t, 7.8) | 2, 4 | 1, 4a | |
| 4 | 124.9 (CH) | 9.07 (d, 8.1) | 3 | 2, 4b, 13a | |
| 4a | 121.4 (C) | ||||
| 4b | 115.2 (C) | ||||
| 4c | 119.5 (C) | ||||
| 5 | 171.2 (C) | ||||
| 6 | — | 11.1 (s) | 4c | ||
| 7 | 170.9 (C) | ||||
| 7a | 120.9 (C) | ||||
| 7b | 116.1 (C) | ||||
| 7c | 122.8 (C) | ||||
| 8 | 124.9 (CH) | 9.25 (d, 7.9) | 9 | 10, 7b, 11a | 10 |
| 9 | 120.6 (CH) | 7.39 (t, 7.3) | 8, 10 | 11, 7c | |
| 10 | 126.9 (CH) | 7.56 (t, 7.8) | 9, 11 | 8, 11a | 8 |
| 11 | 113.7 (CH) | 7.71 (d, 8.1) | 10 | 9, 7c | |
| 11a | 139.9 (C) | ||||
| 12a | 130.5 (C) | ||||
| 12b | 128.8 (C) | ||||
| 13a | 137.7 (C) | ||||
| 1' | 82.6 (CH) | 7.03 (td, 5.8, 2.1) | 2' | 12b 13a, 5' | 3' |
| 2' | 27.4 (CH2) | 2.68~2.71 (m); 2.22 (td, 5.8, 12.8) | 1', 3' | 3', 4' | |
| 3' | 48.3 (CH) | 4.85 (d, 12.8) | 2', 4' | 4' | 1', 6' |
| 4' | 84.0 (CH) | 4.22 (d, 3.0) | 3' | 2', 6', 4'-OCH3 | 11 |
| 5' | 95.2 (C) | ||||
| 6' | 29.2 (CH3) | 2.35 (s) | 4' | 3' | |
| 3'-NCH3 | 30.2 (CH3) | 2.63 (s) | 3', 3'-NCO | ||
| 4'-OCH3 | 60.2 (CH3) | 2.68 (d, 3.0) | 4' | ||
| 3'-NCO | 158.9 (C) | 3'-NCH3 | |||
| NH2 | 6.02 (s) | 3', 3'-NCH3 |
| Position | δC (type) | δH (mult. J in Hz) | COSY | HMBC | NOESY |
|---|---|---|---|---|---|
| 1 | 109.9 (CH) | 7.75 (d, 8.2) | 2 | 3, 4a | |
| 2 | 127.2 (CH) | 7.61 (t, 7.4) | 1, 3 | 4, 13a | |
| 3 | 120.7 (CH) | 7.43 (t, 7.8) | 2, 4 | 1, 4a | |
| 4 | 124.9 (CH) | 9.07 (d, 8.1) | 3 | 2, 4b, 13a | |
| 4a | 121.4 (C) | ||||
| 4b | 115.2 (C) | ||||
| 4c | 119.5 (C) | ||||
| 5 | 171.2 (C) | ||||
| 6 | — | 11.1 (s) | 4c | ||
| 7 | 170.9 (C) | ||||
| 7a | 120.9 (C) | ||||
| 7b | 116.1 (C) | ||||
| 7c | 122.8 (C) | ||||
| 8 | 124.9 (CH) | 9.25 (d, 7.9) | 9 | 10, 7b, 11a | 10 |
| 9 | 120.6 (CH) | 7.39 (t, 7.3) | 8, 10 | 11, 7c | |
| 10 | 126.9 (CH) | 7.56 (t, 7.8) | 9, 11 | 8, 11a | 8 |
| 11 | 113.7 (CH) | 7.71 (d, 8.1) | 10 | 9, 7c | |
| 11a | 139.9 (C) | ||||
| 12a | 130.5 (C) | ||||
| 12b | 128.8 (C) | ||||
| 13a | 137.7 (C) | ||||
| 1' | 82.6 (CH) | 7.03 (td, 5.8, 2.1) | 2' | 12b 13a, 5' | 3' |
| 2' | 27.4 (CH2) | 2.68~2.71 (m); 2.22 (td, 5.8, 12.8) | 1', 3' | 3', 4' | |
| 3' | 48.3 (CH) | 4.85 (d, 12.8) | 2', 4' | 4' | 1', 6' |
| 4' | 84.0 (CH) | 4.22 (d, 3.0) | 3' | 2', 6', 4'-OCH3 | 11 |
| 5' | 95.2 (C) | ||||
| 6' | 29.2 (CH3) | 2.35 (s) | 4' | 3' | |
| 3'-NCH3 | 30.2 (CH3) | 2.63 (s) | 3', 3'-NCO | ||
| 4'-OCH3 | 60.2 (CH3) | 2.68 (d, 3.0) | 4' | ||
| 3'-NCO | 158.9 (C) | 3'-NCH3 | |||
| NH2 | 6.02 (s) | 3', 3'-NCH3 |
| Position | 3 | 4 | ||||
|---|---|---|---|---|---|---|
| δC (type) | δH (mult. J in Hz) | δC (type) | δH (mult. J in Hz) | |||
| 1 | 110.0 (CH) | 7.60 (d, 8.0) | 108.4 (CH) | 7.57 (d, 8.2) | ||
| 2 | 125.2 (CH) | 7.45 (dd, 8.0, 7.4) | 124.9 (CH) | 7.42 (dd, 8.2, 7.4) | ||
| 3 | 119.3 (CH) | 7.63 (dd, 8.2, 7.4) | 118.8 (CH) | 7.27 (dd, 7.9, 7.4) | ||
| 4 | 125.0 (CH) | 9.25 (d, 8.2) | 125.6 (CH) | 9.29 (d, 7.9) | ||
| 4a | 122.9 (C) | 122.5 (C) | ||||
| 4b | 115.0 (C) | 113.5 (C) | ||||
| 4c | 121.0 (C) | 119.0 (C) | ||||
| 5 | 171.1 (C) | 172.3 (C) | ||||
| 6 | 11.1 (s) | 8.54 (s) | ||||
| 7 | 170.9 (C) | 45.4 (CH2) | 4.95 (s) | |||
| 7a | 131.0 (C) | 132.1 (C) | ||||
| 7b | 114.9 (C) | 114.1 (C) | ||||
| 7c | 121.0 (C) | 123.9 (C) | ||||
| 8 | 121.5 (CH) | 9.05 (d, 8.0) | 120.9 (CH) | 7.97 (d, 8.6) | ||
| 9 | 121.1 (CH) | 7.45 (dd, 8.0, 7.4) | 119.8 (CH) | 7.27 (dd, 8.6, 8.2) | ||
| 10 | 127.2 (CH) | 7.63 (dd, 8.5, 7.4) | 124.4 (CH) | 7.45 (dd, 7.9, 8.2) | ||
| 11 | 112.7 (CH) | 8.05 (d, 8.5) | 115.3 (CH) | 7.96 (d, 7.9) | ||
| 11a | 138.9 (C) | 139.5 (C) | ||||
| 12a | 129.8 (C) | 130.0 (C) | ||||
| 12b | 127.3 (C) | 126.7 (C) | ||||
| 13a | 137.6 (C) | 136.4 (C) | ||||
| 1' | 81.0 (CH) | 6.95 (dd, 2.7, 9.3) | 80.0 (CH) | 6.69 (t, 3.7) | ||
| 2' | 27.0 (CH2) | 2.13 (td, 1.8, 2.7); 3.33~3.35 (m) | 29.4 (CH2) | 3.17~3.20 (m); 2.49~2.51 (m) | ||
| 3' | 53.2 (CH) | 4.04~4.06 (m) | 50.1 (CH) | 3.22~3.25 (m) | ||
| 4' | 79.3 (CH) | 4.46 (d, 2.0) | 82.8 (CH) | 4.04 (d, 3.4) | ||
| 5' | 93.3 (C) | 91.1 (C) | ||||
| 6' | 27.8 (CH3) | 2.48 (s) | 29.8 (CH3) | 2.30 (s) | ||
| 3'-NH | 9.01~9.04 (m) | |||||
| 3'-NCH3 | 30.7 (CH3) | 2.20 (s) | 33.4 (CH3) | 1.44 (s) | ||
| 4'-OCH3 | 59.9 (CH3) | 2.71 (s) | 57.3 (CH3) | 3.32 (s) | ||
| Position | 3 | 4 | ||||
|---|---|---|---|---|---|---|
| δC (type) | δH (mult. J in Hz) | δC (type) | δH (mult. J in Hz) | |||
| 1 | 110.0 (CH) | 7.60 (d, 8.0) | 108.4 (CH) | 7.57 (d, 8.2) | ||
| 2 | 125.2 (CH) | 7.45 (dd, 8.0, 7.4) | 124.9 (CH) | 7.42 (dd, 8.2, 7.4) | ||
| 3 | 119.3 (CH) | 7.63 (dd, 8.2, 7.4) | 118.8 (CH) | 7.27 (dd, 7.9, 7.4) | ||
| 4 | 125.0 (CH) | 9.25 (d, 8.2) | 125.6 (CH) | 9.29 (d, 7.9) | ||
| 4a | 122.9 (C) | 122.5 (C) | ||||
| 4b | 115.0 (C) | 113.5 (C) | ||||
| 4c | 121.0 (C) | 119.0 (C) | ||||
| 5 | 171.1 (C) | 172.3 (C) | ||||
| 6 | 11.1 (s) | 8.54 (s) | ||||
| 7 | 170.9 (C) | 45.4 (CH2) | 4.95 (s) | |||
| 7a | 131.0 (C) | 132.1 (C) | ||||
| 7b | 114.9 (C) | 114.1 (C) | ||||
| 7c | 121.0 (C) | 123.9 (C) | ||||
| 8 | 121.5 (CH) | 9.05 (d, 8.0) | 120.9 (CH) | 7.97 (d, 8.6) | ||
| 9 | 121.1 (CH) | 7.45 (dd, 8.0, 7.4) | 119.8 (CH) | 7.27 (dd, 8.6, 8.2) | ||
| 10 | 127.2 (CH) | 7.63 (dd, 8.5, 7.4) | 124.4 (CH) | 7.45 (dd, 7.9, 8.2) | ||
| 11 | 112.7 (CH) | 8.05 (d, 8.5) | 115.3 (CH) | 7.96 (d, 7.9) | ||
| 11a | 138.9 (C) | 139.5 (C) | ||||
| 12a | 129.8 (C) | 130.0 (C) | ||||
| 12b | 127.3 (C) | 126.7 (C) | ||||
| 13a | 137.6 (C) | 136.4 (C) | ||||
| 1' | 81.0 (CH) | 6.95 (dd, 2.7, 9.3) | 80.0 (CH) | 6.69 (t, 3.7) | ||
| 2' | 27.0 (CH2) | 2.13 (td, 1.8, 2.7); 3.33~3.35 (m) | 29.4 (CH2) | 3.17~3.20 (m); 2.49~2.51 (m) | ||
| 3' | 53.2 (CH) | 4.04~4.06 (m) | 50.1 (CH) | 3.22~3.25 (m) | ||
| 4' | 79.3 (CH) | 4.46 (d, 2.0) | 82.8 (CH) | 4.04 (d, 3.4) | ||
| 5' | 93.3 (C) | 91.1 (C) | ||||
| 6' | 27.8 (CH3) | 2.48 (s) | 29.8 (CH3) | 2.30 (s) | ||
| 3'-NH | 9.01~9.04 (m) | |||||
| 3'-NCH3 | 30.7 (CH3) | 2.20 (s) | 33.4 (CH3) | 1.44 (s) | ||
| 4'-OCH3 | 59.9 (CH3) | 2.71 (s) | 57.3 (CH3) | 3.32 (s) | ||
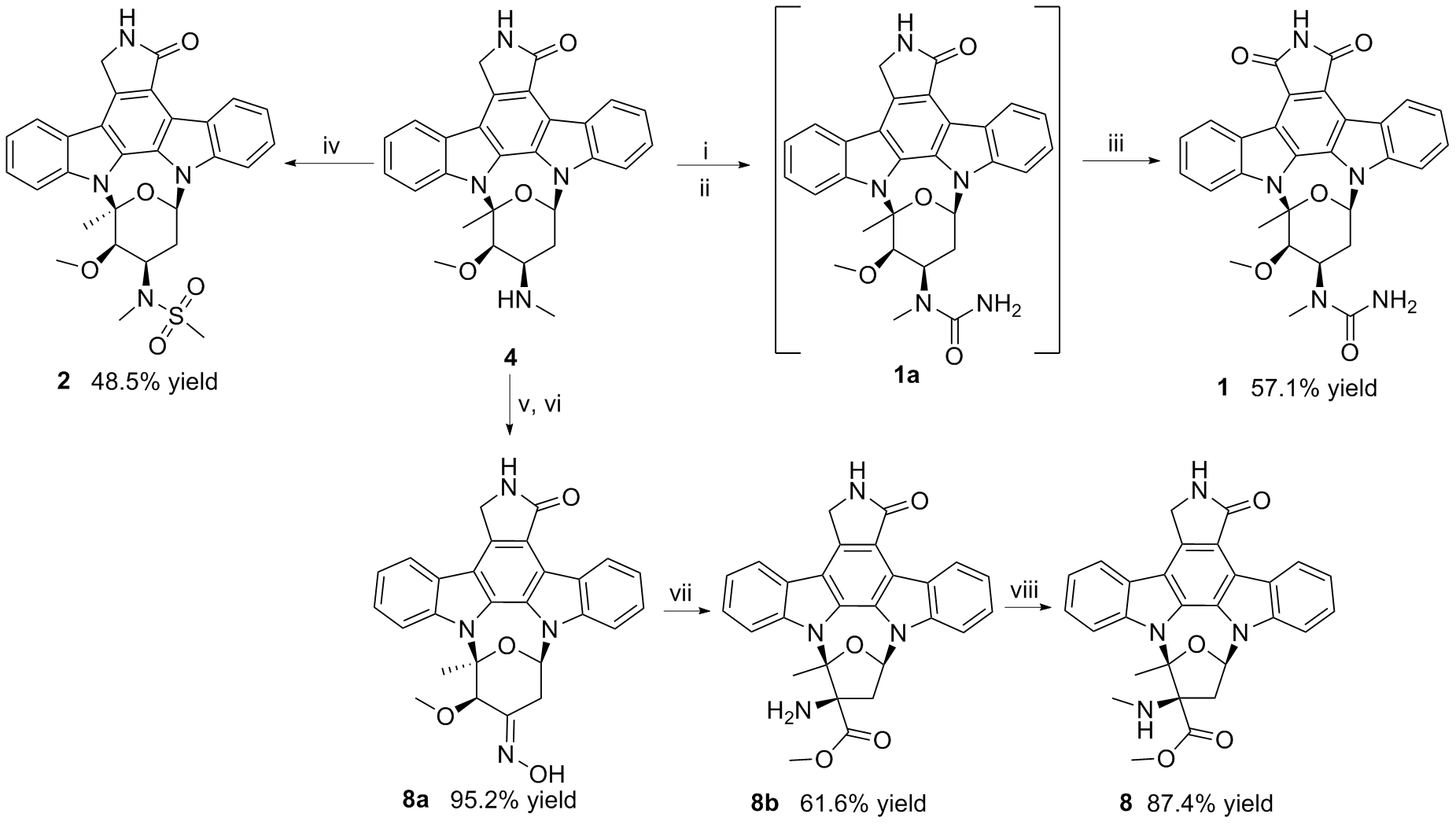
| Position | δC (type) | δH (mult. J in Hz) | COSY | HMBC | NOESY |
|---|---|---|---|---|---|
| 1 | 109.0 (CH) | 7.61 (d, 8.4) | 2 | 3, 4a | 1' |
| 2 | 125.1 (CH) | 7.50 (dd, 8.4, 7.5) | 1, 3 | 4, 13a | |
| 3 | 119.6 (CH) | 7.31 (dd, 8.0, 7.5) | 2, 4 | 1, 4a | |
| 4 | 125.8 (CH) | 9.29 (d, 8.0) | 3 | 2, 4b, 13a | |
| 4a | 122.7 (C) | ||||
| 4b | 115.2 (C) | ||||
| 4c | 119.5 (C) | ||||
| 5 | 172.0 (C) | ||||
| 6 | 8.60 (s) | 5, 7, 7a, 4c | |||
| 7 | 45.5 (CH2) | 5.00 (s) | 5, 4c, 7a, 7b | 8 | |
| 7a | 132.7 (C) | ||||
| 7b | 114.2 (C) | ||||
| 7c | 123.8 (C) | ||||
| 8 | 121.6 (CH) | 8.04 (d,7.6) | 9 | 10, 7a, 11a | 7, 10 |
| 9 | 120.5 (CH) | 7.37 (dd, 7.6, 7.5) | 8, 10 | 11, 7c | |
| 10 | 125.1 (CH) | 7.50 (dd, 8.3, 7.5) | 9, 11 | 8, 11a | 8 |
| 11 | 113.7 (CH) | 8.06 (d, 8.3) | 10 | 9, 7c | |
| 11a | 138.8 (C) | ||||
| 12a | 129.2 (C) | ||||
| 12b | 125.4 (C) | ||||
| 13a | 136.3 (C) | ||||
| 1' | 82.3 (CH) | 7.04 (dd, 8.5, 5.9) | 2' | 12b, 2', 5' | 1, 3' |
| 2' | 26.9 (CH2) | 2.22 (dt, 12.7, 5.9), 2.81~2.83 (m) | 1', 3' | 3' | 4'-CH3 |
| 3' | 51.4 (CH) | 4.40 (dt, 12.7, 3.5) | 2' | 3'-NCH3 | 1' |
| 4' | 85.2 (CH) | 4.29 (brs) | 2', 4'-OCH3 | 4'-OCH3 | |
| 5' | 94.9 (C) | 6' | |||
| 6' | 29.2 (CH3) | 2.36 (s) | 5' | 4' | |
| 3'-NCH3 | 30.4 (CH3) | 2.67 (s) | 3' | ||
| 4'-OCH3 | 60.5 (CH3) | 2.78 (s) | 4' | ||
| 3'-NSO2CH3 | 36.8 (CH3) | 3.12 (s) |
| Position | δC (type) | δH (mult. J in Hz) | COSY | HMBC | NOESY |
|---|---|---|---|---|---|
| 1 | 109.0 (CH) | 7.61 (d, 8.4) | 2 | 3, 4a | 1' |
| 2 | 125.1 (CH) | 7.50 (dd, 8.4, 7.5) | 1, 3 | 4, 13a | |
| 3 | 119.6 (CH) | 7.31 (dd, 8.0, 7.5) | 2, 4 | 1, 4a | |
| 4 | 125.8 (CH) | 9.29 (d, 8.0) | 3 | 2, 4b, 13a | |
| 4a | 122.7 (C) | ||||
| 4b | 115.2 (C) | ||||
| 4c | 119.5 (C) | ||||
| 5 | 172.0 (C) | ||||
| 6 | 8.60 (s) | 5, 7, 7a, 4c | |||
| 7 | 45.5 (CH2) | 5.00 (s) | 5, 4c, 7a, 7b | 8 | |
| 7a | 132.7 (C) | ||||
| 7b | 114.2 (C) | ||||
| 7c | 123.8 (C) | ||||
| 8 | 121.6 (CH) | 8.04 (d,7.6) | 9 | 10, 7a, 11a | 7, 10 |
| 9 | 120.5 (CH) | 7.37 (dd, 7.6, 7.5) | 8, 10 | 11, 7c | |
| 10 | 125.1 (CH) | 7.50 (dd, 8.3, 7.5) | 9, 11 | 8, 11a | 8 |
| 11 | 113.7 (CH) | 8.06 (d, 8.3) | 10 | 9, 7c | |
| 11a | 138.8 (C) | ||||
| 12a | 129.2 (C) | ||||
| 12b | 125.4 (C) | ||||
| 13a | 136.3 (C) | ||||
| 1' | 82.3 (CH) | 7.04 (dd, 8.5, 5.9) | 2' | 12b, 2', 5' | 1, 3' |
| 2' | 26.9 (CH2) | 2.22 (dt, 12.7, 5.9), 2.81~2.83 (m) | 1', 3' | 3' | 4'-CH3 |
| 3' | 51.4 (CH) | 4.40 (dt, 12.7, 3.5) | 2' | 3'-NCH3 | 1' |
| 4' | 85.2 (CH) | 4.29 (brs) | 2', 4'-OCH3 | 4'-OCH3 | |
| 5' | 94.9 (C) | 6' | |||
| 6' | 29.2 (CH3) | 2.36 (s) | 5' | 4' | |
| 3'-NCH3 | 30.4 (CH3) | 2.67 (s) | 3' | ||
| 4'-OCH3 | 60.5 (CH3) | 2.78 (s) | 4' | ||
| 3'-NSO2CH3 | 36.8 (CH3) | 3.12 (s) |
| Position | 5a | 6 | |||
|---|---|---|---|---|---|
| δC (type) | δH (mult. J in Hz) | δC (type) | δH (mult. J in Hz) | ||
| 1 | 108.8 (CH) | 7.65 (d, 8.2) | 109.9 (CH) | 7.69 (d, 8.5) | |
| 2 | 125.5 (CH) | 7.54 (dd, 8.2, 7.0) | 125.2 (CH) | 7.49 (dd, 8.5, 7.5) | |
| 3 | 119.9 (CH) | 7.33 (dd, 7.9, 7.0) | 119.3 (CH) | 7.27 (dd, 7.9, 7.5) | |
| 4 | 125.8 (CH) | 9.31 (d, 7.9) | 125.6 (CH) | 9.47 (d, 7.9) | |
| 4a | 122.8 (C) | 122.3 (C) | |||
| 4b | 114.9 (C) | 117.6 (C) | |||
| 4c | 119.3 (C) | 118.6 (C) | |||
| 5 | 171.9 (C) | 172.3 (C) | |||
| 6 | 8.61 (s) | 8.55 (s) | |||
| 7 | 45.5 (CH2) | 4.98 (dd, 17.6, 24.0) | 45.2 (CH2) | 5.00 (dd, 18.8, 22.1) | |
| 7a | 132.7 (C) | 133.9 (C) | |||
| 7b | 114.4 (C) | 114.9 (C) | |||
| 7c | 123.7 (C) | 121.9 (C) | |||
| 8 | 121.0 (CH) | 8.00 (d, 7.8) | 121.2 (CH) | 8.07 (d, 7.7) | |
| 9 | 120.3 (CH) | 7.33 (dd, 7.8, 7.0) | 119.9 (CH) | 7.31 (dd, 7.7, 7.5) | |
| 10 | 125.0 (CH) | 7.46 (dd, 7.0, 8.5) | 125.2 (CH) | 7.49 (dd, 7.5, 8.0) | |
| 11 | 115.5 (CH) | 7.98 (d, 8.5) | 77.2 (CH) | 7.60 (d, 8.0) | |
| 11a | 140.3 (C) | 139.0 (C) | |||
| 12 | 11.69 (s) | ||||
| 12a | 127.9 (C) | 127.6 (C) | |||
| 12b | 124.7 (C) | 124.6 (C) | |||
| 13a | 136.5 (C) | 140.3 (C) | |||
| 1' | 87.3 (CH) | 6.60 (d, 1.8) | 77.2 (CH) | 6.39 (dd, 2.7, 9.5) | |
| 2' | 71.8 (CH) | 4.13 (dd, 3.1, 1.8) | 67.0 (CH) | 4.49 (dt, 2.5, 9.5) | |
| 3' | 65.7 (CH) | 3.57 (dd, 3.1, 10.5) | 71.7 (CH) | 4.18 (q, 3.4) | |
| 4' | 83.1 (CH) | 4.13 (d, 10.5) | 71.5 (CH) | 4.05 (t, 3.5) | |
| 5' | 95.6 (C) | 76.5 (CH) | 4.48 (q, 6.1) | ||
| 6' | 29.1 (CH3) | 2.39 (s) | 15.4 (CH3) | 1.70 (dd, 2.6, 7.1) | |
| Position | 5a | 6 | |||
|---|---|---|---|---|---|
| δC (type) | δH (mult. J in Hz) | δC (type) | δH (mult. J in Hz) | ||
| 1 | 108.8 (CH) | 7.65 (d, 8.2) | 109.9 (CH) | 7.69 (d, 8.5) | |
| 2 | 125.5 (CH) | 7.54 (dd, 8.2, 7.0) | 125.2 (CH) | 7.49 (dd, 8.5, 7.5) | |
| 3 | 119.9 (CH) | 7.33 (dd, 7.9, 7.0) | 119.3 (CH) | 7.27 (dd, 7.9, 7.5) | |
| 4 | 125.8 (CH) | 9.31 (d, 7.9) | 125.6 (CH) | 9.47 (d, 7.9) | |
| 4a | 122.8 (C) | 122.3 (C) | |||
| 4b | 114.9 (C) | 117.6 (C) | |||
| 4c | 119.3 (C) | 118.6 (C) | |||
| 5 | 171.9 (C) | 172.3 (C) | |||
| 6 | 8.61 (s) | 8.55 (s) | |||
| 7 | 45.5 (CH2) | 4.98 (dd, 17.6, 24.0) | 45.2 (CH2) | 5.00 (dd, 18.8, 22.1) | |
| 7a | 132.7 (C) | 133.9 (C) | |||
| 7b | 114.4 (C) | 114.9 (C) | |||
| 7c | 123.7 (C) | 121.9 (C) | |||
| 8 | 121.0 (CH) | 8.00 (d, 7.8) | 121.2 (CH) | 8.07 (d, 7.7) | |
| 9 | 120.3 (CH) | 7.33 (dd, 7.8, 7.0) | 119.9 (CH) | 7.31 (dd, 7.7, 7.5) | |
| 10 | 125.0 (CH) | 7.46 (dd, 7.0, 8.5) | 125.2 (CH) | 7.49 (dd, 7.5, 8.0) | |
| 11 | 115.5 (CH) | 7.98 (d, 8.5) | 77.2 (CH) | 7.60 (d, 8.0) | |
| 11a | 140.3 (C) | 139.0 (C) | |||
| 12 | 11.69 (s) | ||||
| 12a | 127.9 (C) | 127.6 (C) | |||
| 12b | 124.7 (C) | 124.6 (C) | |||
| 13a | 136.5 (C) | 140.3 (C) | |||
| 1' | 87.3 (CH) | 6.60 (d, 1.8) | 77.2 (CH) | 6.39 (dd, 2.7, 9.5) | |
| 2' | 71.8 (CH) | 4.13 (dd, 3.1, 1.8) | 67.0 (CH) | 4.49 (dt, 2.5, 9.5) | |
| 3' | 65.7 (CH) | 3.57 (dd, 3.1, 10.5) | 71.7 (CH) | 4.18 (q, 3.4) | |
| 4' | 83.1 (CH) | 4.13 (d, 10.5) | 71.5 (CH) | 4.05 (t, 3.5) | |
| 5' | 95.6 (C) | 76.5 (CH) | 4.48 (q, 6.1) | ||
| 6' | 29.1 (CH3) | 2.39 (s) | 15.4 (CH3) | 1.70 (dd, 2.6, 7.1) | |
| Position | 7 | 8 | |||
|---|---|---|---|---|---|
| δC (type) | δH (mult. J in Hz) | δC (type) | δH (mult. J in Hz) | ||
| 1 | 109.2 (CH) | 7.94 (d, 8.5) | 109.2 (CH) | 7.92 (d, 8.3) | |
| 2 | 125.5 (CH) | 7.50 (dd, 8.5, 7.4) | 125.4 (CH) | 7.48 (dd, 8.3, 7.2) | |
| 3 | 119.6 (CH) | 7.29 (dd, 7.4, 8.0) | 119.6 (CH) | 7.29 (dd, 7.2, 8.0) | |
| 4 | 125.7 (CH) | 9.22 (d, 8.0) | 125.6 (CH) | 9.21 (d, 8.0) | |
| 4a | 122.6 (C) | 122.6 (C) | |||
| 4b | 115.8 (C) | 115.9 (C) | |||
| 4c | 120.5 (C) | 119.7 (C) | |||
| 5 | 171.8 (C) | 171.8 (C) | |||
| 6 | 8.65 (s) | 8.66 (s) | |||
| 7 | 45.5 (CH2) | 5.01 (dd, 17.8, 23.0) | 45.6 (CH2) | 5.00 (dd, 16.0, 20.0) | |
| 7a | 133.0 (C) | 133.0 (C) | |||
| 7b | 114.9 (C) | 114.7 (C) | |||
| 7c | 124.0 (C) | 124.0 (C) | |||
| 8 | 121.3 (CH) | 8.07 (d, 7.6) | 121.4 (CH) | 8.05 (d, 7.8) | |
| 9 | 119.6 (CH) | 7.37 (dd, 7.4, 7.6) | 120.6 (CH) | 7.36 (dd, 7.4, 7.8) | |
| 10 | 125.1 (CH) | 7.50 (dd, 8.5, 7.4) | 125.7 (CH) | 7.49 (dd, 8.5, 7.4) | |
| 11 | 114.6 (CH) | 7.91 (d, 8.5) | 114.9 (CH) | 8.09 (d, 8.5) | |
| 11a | 140.0 (C) | 139.7 (C) | |||
| 12a | 128.4 (C) | 128.5 (C) | |||
| 12b | 124.2 (C) | 124.2 (C) | |||
| 13a | 136.9 (C) | 136.8 (C) | |||
| 1' | 85.1 (CH) | 7.15 (dd, 5.0, 7.1) | 85.9 (CH) | 7.20 (dd, 5.1, 7.3) | |
| 2' | 42.6 (CH2) | 2.02 (dd, 5.0, 14); 3.40 (t, 7.1) | 40.1 (CH2) | 2.03 (dd, 5.1, 13.8); 3.43 (dd, 7.3, 13.8) | |
| 3' | 85.0 (C) | 78.1 (C) | |||
| 4' | 99.4 (C) | 100.5 (C) | |||
| 5' | 172.9 (C) | 173.0 (C) | |||
| 6' | 22.9 (CH3) | 2.16 (s) | 23.9 (CH3) | 2.17 (s) | |
| 5'-OCH3 | 52.8 (CH3) | 3.94 (s) | 53.0 (CH3) | 3.94 (s) | |
| Position | 7 | 8 | |||
|---|---|---|---|---|---|
| δC (type) | δH (mult. J in Hz) | δC (type) | δH (mult. J in Hz) | ||
| 1 | 109.2 (CH) | 7.94 (d, 8.5) | 109.2 (CH) | 7.92 (d, 8.3) | |
| 2 | 125.5 (CH) | 7.50 (dd, 8.5, 7.4) | 125.4 (CH) | 7.48 (dd, 8.3, 7.2) | |
| 3 | 119.6 (CH) | 7.29 (dd, 7.4, 8.0) | 119.6 (CH) | 7.29 (dd, 7.2, 8.0) | |
| 4 | 125.7 (CH) | 9.22 (d, 8.0) | 125.6 (CH) | 9.21 (d, 8.0) | |
| 4a | 122.6 (C) | 122.6 (C) | |||
| 4b | 115.8 (C) | 115.9 (C) | |||
| 4c | 120.5 (C) | 119.7 (C) | |||
| 5 | 171.8 (C) | 171.8 (C) | |||
| 6 | 8.65 (s) | 8.66 (s) | |||
| 7 | 45.5 (CH2) | 5.01 (dd, 17.8, 23.0) | 45.6 (CH2) | 5.00 (dd, 16.0, 20.0) | |
| 7a | 133.0 (C) | 133.0 (C) | |||
| 7b | 114.9 (C) | 114.7 (C) | |||
| 7c | 124.0 (C) | 124.0 (C) | |||
| 8 | 121.3 (CH) | 8.07 (d, 7.6) | 121.4 (CH) | 8.05 (d, 7.8) | |
| 9 | 119.6 (CH) | 7.37 (dd, 7.4, 7.6) | 120.6 (CH) | 7.36 (dd, 7.4, 7.8) | |
| 10 | 125.1 (CH) | 7.50 (dd, 8.5, 7.4) | 125.7 (CH) | 7.49 (dd, 8.5, 7.4) | |
| 11 | 114.6 (CH) | 7.91 (d, 8.5) | 114.9 (CH) | 8.09 (d, 8.5) | |
| 11a | 140.0 (C) | 139.7 (C) | |||
| 12a | 128.4 (C) | 128.5 (C) | |||
| 12b | 124.2 (C) | 124.2 (C) | |||
| 13a | 136.9 (C) | 136.8 (C) | |||
| 1' | 85.1 (CH) | 7.15 (dd, 5.0, 7.1) | 85.9 (CH) | 7.20 (dd, 5.1, 7.3) | |
| 2' | 42.6 (CH2) | 2.02 (dd, 5.0, 14); 3.40 (t, 7.1) | 40.1 (CH2) | 2.03 (dd, 5.1, 13.8); 3.43 (dd, 7.3, 13.8) | |
| 3' | 85.0 (C) | 78.1 (C) | |||
| 4' | 99.4 (C) | 100.5 (C) | |||
| 5' | 172.9 (C) | 173.0 (C) | |||
| 6' | 22.9 (CH3) | 2.16 (s) | 23.9 (CH3) | 2.17 (s) | |
| 5'-OCH3 | 52.8 (CH3) | 3.94 (s) | 53.0 (CH3) | 3.94 (s) | |
| Position | 9 | 10a | 11a | |||||
|---|---|---|---|---|---|---|---|---|
| δC (type) | δH (mult. J in Hz) | δC (type) | δH (mult. J in Hz) | δC (type) | δH (mult. J in Hz) | |||
| 1 | 109.5 (CH) | 7.95 (d, 8.5) | 109.7 (CH) | 8.02 (d, 8.4) | 110.5 (CH) | 8.14 (d, 8.5) | ||
| 2 | 125.5 (CH) | 7.52 (dd, 8.5, 7.3) | 125.4 (CH) | 7.50 (dd, 8.4, 7.3) | 126.9 (CH) | 7.60 (dd, 7.6, 8.5) | ||
| 3 | 120.0 (CH) | 7.32 (dd, 7.9, 7.3) | 119.7 (CH) | 7.29 (dd, 7.3, 7.9) | 120.2 (CH) | 7.37 (dd, 7.6, 8.0) | ||
| 4 | 125.9 (CH) | 9.47 (d,7.9) | 125.7 (CH) | 9.46 (d, 7.9) | 124.6 (CH) | 9.10 (d, 8.0) | ||
| 4a | 122.3 (C) | 122.2 (C) | 121.1 (C) | |||||
| 4b | 117.5 (C) | 117.3 (C) | 117.3 (C) | |||||
| 4c | 118.6 (C) | 118.5 (C) | 119.2 (C) | |||||
| 5 | 172.2 (C) | 172.3 (C) | 171.1 (C) | |||||
| 6 | 8.58 (s) | 8.55 (s) | 11.11 (s) | |||||
| 7 | 45.2 (CH2) | 5.00 (dd, 16.9, 20.3) | 45.2 (CH2) | 5.00 (dd, 17.4, 20.2) | 171.2 (C) | |||
| 7a | 134.3 (C) | 134.1 (C) | 120.8 (C) | |||||
| 7b | 115.1 (C) | 114.9 (C) | 116.5 (C) | |||||
| 7c | 121.8 (C) | 121.8 (C) | 121.0 (C) | |||||
| 8 | 121.2 (CH) | 8.08 (d, 7.7) | 121.2 (CH) | 8.07 (d, 7.9) | 124.7 (CH) | 9.20 (d, 8.1) | ||
| 9 | 120.0 (CH) | 7.32 (dd, 7.7, 7.5) | 119.8 (CH) | 7.32 (dd, 7.9, 7.4) | 120.9 (CH) | 7.42 (dd, 7.4, 8.1)) | ||
| 10 | 125.1 (CH) | 7.49 (dd, 7.2, 8.8) | 125.0 (CH) | 7.50 (dd, 7.4, 8.1) | 127.1 (CH) | 7.61 (dd, 7.4, 8.3) | ||
| 11 | 113.6 (CH) | 8.01 (d, 8.5) | 111.7 (CH) | 7.71 (d, 8.1) | 111.8 (CH) | 7.73 (d, 8.3) | ||
| 11a | 139.2 (C) | 139.3 (C) | 139.8 (C) | |||||
| 12 | 11.79 (s) | 12.13 (s) | 12.34 (s) | |||||
| 12a | 127.2 (C) | 127.4 (C) | 127.6 (C) | |||||
| 12b | 124.0 (C) | 124.2 (C) | 128.7 (C) | |||||
| 13a | 138.5 (C) | 138.6 (C) | 140.4 (C) | |||||
| 1' | 75.1 (CH) | 6.71 (dd, 11.2, 4.1) | 75.9 (CH) | 6.73 (dd, 4.3, 11.3) | 76.1 (CH) | 6.77 (dd (3.3, 11.5) | ||
| 2' | 30.7 (CH2) | 2.02 (dt, 4.1, 12.7); 2.55 (dd, 11.2, 12.7) | 32.2 (CH2) | 1.80 (dt, 4.3, 12.9); 2.52 (d, 12.9) | 32.0 (CH2) | 1.85 (dt, 3.3, 12.4); 2.50 (m) | ||
| 3' | 45.8 (CH) | 4.12~4.15 (m) | 44.3 (CH) | 4.70 (dd, 3.3, 7.7) | 44.1 (CH) | 4.71 (dd, 5.7, 7.8) | ||
| 4' | 66.1 (CH) | 4.08~4.10 (m) | 68.2 (CH) | 3.92 (d, 3.3) | 68.2 (CH) | 3.92 (brs) | ||
| 5' | 76.5 (CH) | 4.62 (q, 6.9) | 76.7 (CH) | 4.52 (q, 6.7) | 77.0 (CH) | 4.53 (q, 7.1) | ||
| 6' | 14.0 (CH3) | 1.60 (d, 7.2) | 14.4 (CH3) | 1.61 (d, 7.1) | 14.3 (CH3) | 1.61 (d, 7.1) | ||
| 3'-NH | 8.19~8.21 (m, 2H, NH2) | 7.92 (d, 7.7) | 7.93 (d, 7.8) | |||||
| 4'-OH | 7.67 (m) | 6.96 (s) | 7.02 (s) | |||||
| Position | 9 | 10a | 11a | |||||
|---|---|---|---|---|---|---|---|---|
| δC (type) | δH (mult. J in Hz) | δC (type) | δH (mult. J in Hz) | δC (type) | δH (mult. J in Hz) | |||
| 1 | 109.5 (CH) | 7.95 (d, 8.5) | 109.7 (CH) | 8.02 (d, 8.4) | 110.5 (CH) | 8.14 (d, 8.5) | ||
| 2 | 125.5 (CH) | 7.52 (dd, 8.5, 7.3) | 125.4 (CH) | 7.50 (dd, 8.4, 7.3) | 126.9 (CH) | 7.60 (dd, 7.6, 8.5) | ||
| 3 | 120.0 (CH) | 7.32 (dd, 7.9, 7.3) | 119.7 (CH) | 7.29 (dd, 7.3, 7.9) | 120.2 (CH) | 7.37 (dd, 7.6, 8.0) | ||
| 4 | 125.9 (CH) | 9.47 (d,7.9) | 125.7 (CH) | 9.46 (d, 7.9) | 124.6 (CH) | 9.10 (d, 8.0) | ||
| 4a | 122.3 (C) | 122.2 (C) | 121.1 (C) | |||||
| 4b | 117.5 (C) | 117.3 (C) | 117.3 (C) | |||||
| 4c | 118.6 (C) | 118.5 (C) | 119.2 (C) | |||||
| 5 | 172.2 (C) | 172.3 (C) | 171.1 (C) | |||||
| 6 | 8.58 (s) | 8.55 (s) | 11.11 (s) | |||||
| 7 | 45.2 (CH2) | 5.00 (dd, 16.9, 20.3) | 45.2 (CH2) | 5.00 (dd, 17.4, 20.2) | 171.2 (C) | |||
| 7a | 134.3 (C) | 134.1 (C) | 120.8 (C) | |||||
| 7b | 115.1 (C) | 114.9 (C) | 116.5 (C) | |||||
| 7c | 121.8 (C) | 121.8 (C) | 121.0 (C) | |||||
| 8 | 121.2 (CH) | 8.08 (d, 7.7) | 121.2 (CH) | 8.07 (d, 7.9) | 124.7 (CH) | 9.20 (d, 8.1) | ||
| 9 | 120.0 (CH) | 7.32 (dd, 7.7, 7.5) | 119.8 (CH) | 7.32 (dd, 7.9, 7.4) | 120.9 (CH) | 7.42 (dd, 7.4, 8.1)) | ||
| 10 | 125.1 (CH) | 7.49 (dd, 7.2, 8.8) | 125.0 (CH) | 7.50 (dd, 7.4, 8.1) | 127.1 (CH) | 7.61 (dd, 7.4, 8.3) | ||
| 11 | 113.6 (CH) | 8.01 (d, 8.5) | 111.7 (CH) | 7.71 (d, 8.1) | 111.8 (CH) | 7.73 (d, 8.3) | ||
| 11a | 139.2 (C) | 139.3 (C) | 139.8 (C) | |||||
| 12 | 11.79 (s) | 12.13 (s) | 12.34 (s) | |||||
| 12a | 127.2 (C) | 127.4 (C) | 127.6 (C) | |||||
| 12b | 124.0 (C) | 124.2 (C) | 128.7 (C) | |||||
| 13a | 138.5 (C) | 138.6 (C) | 140.4 (C) | |||||
| 1' | 75.1 (CH) | 6.71 (dd, 11.2, 4.1) | 75.9 (CH) | 6.73 (dd, 4.3, 11.3) | 76.1 (CH) | 6.77 (dd (3.3, 11.5) | ||
| 2' | 30.7 (CH2) | 2.02 (dt, 4.1, 12.7); 2.55 (dd, 11.2, 12.7) | 32.2 (CH2) | 1.80 (dt, 4.3, 12.9); 2.52 (d, 12.9) | 32.0 (CH2) | 1.85 (dt, 3.3, 12.4); 2.50 (m) | ||
| 3' | 45.8 (CH) | 4.12~4.15 (m) | 44.3 (CH) | 4.70 (dd, 3.3, 7.7) | 44.1 (CH) | 4.71 (dd, 5.7, 7.8) | ||
| 4' | 66.1 (CH) | 4.08~4.10 (m) | 68.2 (CH) | 3.92 (d, 3.3) | 68.2 (CH) | 3.92 (brs) | ||
| 5' | 76.5 (CH) | 4.62 (q, 6.9) | 76.7 (CH) | 4.52 (q, 6.7) | 77.0 (CH) | 4.53 (q, 7.1) | ||
| 6' | 14.0 (CH3) | 1.60 (d, 7.2) | 14.4 (CH3) | 1.61 (d, 7.1) | 14.3 (CH3) | 1.61 (d, 7.1) | ||
| 3'-NH | 8.19~8.21 (m, 2H, NH2) | 7.92 (d, 7.7) | 7.93 (d, 7.8) | |||||
| 4'-OH | 7.67 (m) | 6.96 (s) | 7.02 (s) | |||||
| 培养基名称 | 配方 |
|---|---|
| A1 | 可溶性淀粉10 g, 酵母浸膏4 g, 蛋白胨2 g, Fe2(SO4)3•4H2O 0.04 g, KBr 0.1 g, 海水1 L, 自然pH |
| Kazuo | 可溶性淀粉25 g, 酵母浸膏2 g, 豆粉15 g, CaCO3 2 g, Amberlite XAD-16N 10 g, 海水1 L, 自然pH |
| LB | 胰蛋白胨10 g, NaCl 5 g, 酵母浸膏5 g, 自来水1 L, 自然pH |
| YPD | 酵母浸膏10 g, 蛋白胨20 g, 葡萄糖20 g, 海水1 L, 自然pH |
| 分离培养基 | 可溶性淀粉10 g, 干酪素0.3 g, 硝酸钾2 g, 七水合硫酸镁0.05 g, 氯化钠2.0 g, 磷酸氢二钾2.0 g, 碳酸钙0.02 g, 七水合硫酸亚铁0.01 g, 海水500 L, 水500 L, 琼脂20 g, 重铬酸钾50 mg, pH 7.2~7.4 |
| 纯化培养基 | 葡萄糖4.0 g, 酵母膏4.0 g, 麦芽浸粉10.0 g, 海盐27.0 g, 水1000 mL, pH 7.0 |
| 培养基名称 | 配方 |
|---|---|
| A1 | 可溶性淀粉10 g, 酵母浸膏4 g, 蛋白胨2 g, Fe2(SO4)3•4H2O 0.04 g, KBr 0.1 g, 海水1 L, 自然pH |
| Kazuo | 可溶性淀粉25 g, 酵母浸膏2 g, 豆粉15 g, CaCO3 2 g, Amberlite XAD-16N 10 g, 海水1 L, 自然pH |
| LB | 胰蛋白胨10 g, NaCl 5 g, 酵母浸膏5 g, 自来水1 L, 自然pH |
| YPD | 酵母浸膏10 g, 蛋白胨20 g, 葡萄糖20 g, 海水1 L, 自然pH |
| 分离培养基 | 可溶性淀粉10 g, 干酪素0.3 g, 硝酸钾2 g, 七水合硫酸镁0.05 g, 氯化钠2.0 g, 磷酸氢二钾2.0 g, 碳酸钙0.02 g, 七水合硫酸亚铁0.01 g, 海水500 L, 水500 L, 琼脂20 g, 重铬酸钾50 mg, pH 7.2~7.4 |
| 纯化培养基 | 葡萄糖4.0 g, 酵母膏4.0 g, 麦芽浸粉10.0 g, 海盐27.0 g, 水1000 mL, pH 7.0 |
| [1] |
|
|
( 田伟生, 史勇, 化学进展, 2010, 22, 537.)
|
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
|
( 罗云, 高谕康, 燕鹏程, 朱伟明, 有机化学, 2022, 42, 2840.)
doi: 10.6023/cjoc202204035 |
|
| [8] |
|
|
( 刘海珊, 朱国良, 赵水鸽, 付鹏, 朱伟明, 有机化学, 2019, 39, 507.)
doi: 10.6023/cjoc201806045 |
|
| [9] |
|
|
( 梅显贵, 王立平, 王冬阳, 范杰, 朱伟明, 有机化学, 2017, 37, 2352.)
doi: 10.6023/cjoc201703048 |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
|
( 林思敏, 徐广进, 高海, 王聪, 朱伟明, 中国海洋药物, 2023, 42, 36.)
|
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
pmid: 1556009 |
| [25] |
|
|
|
|
| [26] |
|
| [27] |
pmid: 3759659 |
| [28] |
|
| [29] |
|
| [30] |
pmid: 8968403 |
| [31] |
|
| [32] |
|
| [33] |
|
| [34] |
doi: 10.1021/acs.jnatprod.8b00103 pmid: 30106291 |
| [35] |
|
| [36] |
|
|
( 王聪, 王立平, 范杰, 孙坤来, 朱伟明, 有机化学, 2017, 37, 658.)
doi: 10.6023/cjoc201609021 |
| [1] | Jiaqiang Yang, Xurong Zhou, Yangmi Chen, Huixian She. Synthesis and Antibacterial Activities of Osthole Derivatives Containing Phosphonateptide Fragment [J]. Chinese Journal of Organic Chemistry, 2025, 45(4): 1306-1314. |
| [2] | Lingdong Li, Weilun Zhang, Pengfei Liu, Zijie Zhou, Hao Zhou, Zhongtian Du. Synthesis of Gemini-Quaternary Ammonium N-Chloramine Biocides for Antibacterial Applications [J]. Chinese Journal of Organic Chemistry, 2024, 44(6): 2041-2048. |
| [3] | Limin Wang, Xiaoyu Song, Senxiang Cheng, Tong Chen, Deyun Cui. Synthesis and Activity Study of 1,2-Benzisothiazol-3-one Derivatives [J]. Chinese Journal of Organic Chemistry, 2024, 44(6): 1897-1906. |
| [4] | Jiaqiang Yang, Xuejiao Wu, Zicong Lu, Yangmi Chen, Huixian She, Haijun Liu. Design, Synthesis and Antibacterial Activities of Osthole Derivatives Containing Thiazole Fragment [J]. Chinese Journal of Organic Chemistry, 2024, 44(11): 3541-3549. |
| [5] | Haibo Huo, Guixia Li, Shijun Wang, Chun Han, Baojun Shi, Jian Li. Novel γ-Carboline Derivatives as Antibacterial Agents: Synthesis and Antibacterial Evaluation [J]. Chinese Journal of Organic Chemistry, 2024, 44(1): 204-215. |
| [6] | Weizhong Ding, Bingwen Zhang, Yanqing Xue, Yuqi Lin, Zhijun Tang, Jing Wang, Wenchao Yang, Xiaofeng Wang, Wen Liu. A New Polyketide from Fusarium graminearum [J]. Chinese Journal of Organic Chemistry, 2023, 43(9): 3319-3322. |
| [7] | Yifang Chen, Xin Luo, Yu Wang, Zhifu Xing, Ju Peng, Jixiang Chen. Design, Synthesis and Antibacterial Activity of 1,3,4-Oxadiazole Sufones Containing Sulfonamide Structure [J]. Chinese Journal of Organic Chemistry, 2023, 43(1): 274-284. |
| [8] | Dan Huang, Xuhua Nong, Jianni Yang, Chen Li, Changri Han, Guangying Chen, Xiaoping Song, Zhenfan Sun, Yang Hui, Wenhao Chen. Study on the Secondary Metabolites of the Endophytic Fungus Aspergillus terreus HQ100X-1 in Scutellaria formosana [J]. Chinese Journal of Organic Chemistry, 2022, 42(9): 2961-2966. |
| [9] | Huanhuan Wang, Pu Yang, Hongjin Zhai, Shuo Zhang, Yaquan Cao, Yingxue Yang, Chunli Wu. Design, Synthesis and Activity Evaluation of New Tylosin Derivatives [J]. Chinese Journal of Organic Chemistry, 2022, 42(2): 557-572. |
| [10] | Ali Dai, Renfeng Zhang, Chuanhui Li, Lijiao Yu, Ya Wang, Jian Wu. Synthesis and Biological Activity of N-Cyano Sulfonimide Derivatives Bearing Trifluoromethyl Pyridinamide [J]. Chinese Journal of Organic Chemistry, 2021, 41(9): 3633-3642. |
| [11] | Feng He, Shengxin Guo, Ali Dai, Renfeng Zhang, Jian Wu. Synthesis, Characterization, and Biological Activity of Novel Amide Derivatives Containing Trifluoromethylpyridine Moieties [J]. Chinese Journal of Organic Chemistry, 2021, 41(8): 3303-3311. |
| [12] | Shao Lihui, Gan Yiyuan, Hou Mi, Tao Shilin, Zhang Liqiong, Wang Zhenchao, Ouyang Guiping. Design, Synthesis and Biological Activity of Quinazolinone Derivatives Containing Hydrazone Structural Units [J]. Chinese Journal of Organic Chemistry, 2020, 40(7): 1975-1982. |
| [13] | Li Yongkun, Tang Yanling, Li Minxin, Yang Xiaobi, Gao Hui, Mao Zewei. Synthesis and Antibacterial Activity of Thienyl Chalcone Derivatives Bearing Piperazine Moiety [J]. Chinese Journal of Organic Chemistry, 2020, 40(1): 108-114. |
| [14] | Fu Lin, Sun Bingxia, Zhai Jiadai, Li Yuanyuan, Liu Xinqiang, Song Ru, Shi Guanqun, Li Jiaona, Song Yuanxia, Sang Feng. Synthesis and Antibacterial Activity Study of Natural 5'-Hydroxyisoprenyl Chalcones [J]. Chinese Journal of Organic Chemistry, 2020, 40(1): 201-208. |
| [15] | He Wenjing, Liu Denyue, Gan Xiuhai, Zhang Jian, Liu Zhengjun, Yi Chongfen, Song Bao'an. Synthesis and Biological Activity of Novel 1,3,4-Thiadiazolo[3,2-a]pyrimidinone Mesoionic Derivatives [J]. Chin. J. Org. Chem., 2019, 39(8): 2287-2294. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||