Chinese Journal of Organic Chemistry ›› 2025, Vol. 45 ›› Issue (11): 4108-4127.DOI: 10.6023/cjoc202503030 Previous Articles Next Articles
REVIEWS
任志明a, 谢立娟b,c, 晋静c, 吴玉洁c, 刘迪c,*( ), 李久艳a,*(
), 李久艳a,*( )
)
收稿日期:2025-03-28
修回日期:2025-05-17
发布日期:2025-06-19
基金资助:
Zhiming Rena, Lijuan Xieb,c, Jing Jinc, Yujie Wuc, Di Liuc,*( ), Jiuyan Lia,*(
), Jiuyan Lia,*( )
)
Received:2025-03-28
Revised:2025-05-17
Published:2025-06-19
Contact:
*E-mail: liudi@dlut.edu.cn; jiuyanli@dlut.edu.cn
Supported by:Share
Zhiming Ren, Lijuan Xie, Jing Jin, Yujie Wu, Di Liu, Jiuyan Li. Recent Advances in Multi-resonance Luminescent Materials Based on Indolo[3,2,1-jk]carbazole[J]. Chinese Journal of Organic Chemistry, 2025, 45(11): 4108-4127.
| Compound | λem/nm (Sol/film) | ФPL/% (Sol/film) | ES1/ET1/∆EST/eV | HOMO/LUMO/Eg/eV | Td/Tg/℃ | Ref. |
|---|---|---|---|---|---|---|
| tDIDCz | 393/— | 60.0/— | 3.15/2.71/0.44 | -5.98/-2.59/3.39 | —/— | [ |
| m-FLDID | 400/404 | 71.0/— | 3.08/2.72/0.36 | -5.94/-2.62/3.32 | —/— | [ |
| m-FDIDCz-2DPA | 451a/458 | —/66.7 | —/—/— | -5.14/-2.39/2.75b | 545/— | [ |
| DICz | 446a/451 | 99/93 | —/—/— | —/—/— | —/— | [ |
| mMes2DICz | 445a/451 | 99/99 | —/—/— | -5.28/-2.77/2.51 | 455/— | |
| mtPh2DICz | 457a461 | 99/94 | —/—/— | -5.45/-2.69/3.02 | 496/— | |
| Cz-DICz | 457/460 | 98.6/98.4 | 2.71/2.37/0.34 | −5.55/−2.59/2.96/ | —/— | [ |
| DiICzMes4 | —/— | —/82 | 2.82/2.56/0.26 | -5.45/-2.43/3.02 | 450/— | [ |
| p-FLDID | 445/449 | 97.3/82.8 | 2.76/2.57/0.19 | -5.76/-2.90/2.85 | 0.32/0.65 | [ |
| BisICz | 436/442 | 81/76 | 2.84/2.55/0.29 | -5.80/-3.00/2.80 | 0.81/— | [ |
| tBisICz | 436/442 | 95/77 | 2.84/2.55/0.29 | -5.70/-2.90/2.80 | 0.48/0.47 | |
| tPBisICz | 445/450 | 91/78 | 2.81/2.54/0.27 | -5.90/-3.20/2.70 | 0.35/0.56 | |
| pICz-PPO | 438/— | 42/— | 2.82/2.47/0.35 | -5.34/-2.54/2.80 | 514/— | [ |
| pICz-2PPO | 439/— | 42/— | 2.82/2.46/0.36 | -5.37/-2.57/2.80 | 552/— | |
| tBisICz-DPA | 444/— | 96/— | 2.79/2.52/0.28 | -5.65/-2.89/2.76 | 0.45/0.51 | [ |
| tBisICz-PhCz | 440/— | 91/— | 2.82/2.51/0.31 | -5.67/-2.89/2.78 | 0.45/0.46 | |
| pICz | 441a/— | 88/— | 2.81/2.52/0.29 | -5.29/-2.5/2.79 | —/— | [ |
| pICz-TPA | 447a/— | 90/— | 2.77/2.45/0.32 | -5.14/-2.39/2.75 | —/— | |
| DPA-bisICZ | 500/505 | 78/92 | 2.48/2.34/0.14 | -4.91/-2.43/2.48 | 0.78/0.14 | [ |
| tBuDPA-bisICZ | 508/515 | 82/93 | 2.44/2.29/0.15 | -4.82/-2.39/2.43 | 0.79/0.14 | |
| MeODPA-bisICZ | 523/532 | 83/90 | 2.35/2.19/0.16 | -4.68/-2.33/2.35 | 0.78/0.12 | |
| 2,5-tDPAtDIDCz | —/438c | 88/— | 2.83/2.65/0.18 | -5.55/-2.74/2.81 | 0.26/0.62 | [ |
| 1,6-tDPAtDIDCz | —/415c | 92/— | 2.99/2.58/0.41 | -5.54/-2.66/2.88 | 0.92/— | |
| tBu-TiTAT | 452a/454 | —/76 | 2.90/2.54/0.36 | -5.39/-2.64/2.75b | 468/— | [ |
| tBu-TTAT | 425a/430 | —/36 | 3.13/2.60/0.53 | -5.30/-2.55/2.75b | 409/— | |
| α-NAICZ | 598/— | 97/— | 2.07/1.69/0.38 | -4.69/-2.56/2.13 | 431/— | [ |
| α-EtNAICZ | 620/— | 90/— | 2.00/1.69/0.31 | -4.53/-2.48/2.05 | 412/— | |
| NBisICz | 445/457 | —/63 | 2.79/2.37/0.42 | -5.68/-2.92/2.76b | 525/— | [ |
| NBisICz-PCz | 445/454 | —/64 | 2.79/2.37/0.42 | -5.65/-2.90/2.75b | 541/— | |
| NBisICz-DPA | 451/460 | —/64 | 2.75/2.37/0.37 | -5.71/-2.98/2.73b | 520/— | |
| ββICZ | 502/— | 99/— | 2.47/1.94/0.53 | -5.35/-2.85/2.50 | 518/— | [ |
| αβICZ | 472/— | 56/— | 2.64/2.11/0.53 | -5.55/-2.82/2.73 | —/— | |
| ββCNICZ | 501/— | 95/— | 2.47/1.93/0.54 | -5.51/-3.00/2.51 | 550/— | |
| t-mp3ICz | 436c | 99/— | 2.84/2.54/0.30 | -5.63/-2.84/2.79 | 0.49/0.50 | [ |
| p-mp3ICz | 444c | 97/— | 2.80/2.54/0.26 | -5.63/-2.87/2.76 | 0.35/0.62 | |
| t3IDCz | —/459c | 92/— | 2.70/2.49/0.21 | -5.50/-2.85/2.65 | 0.28/0.64 | [ |
| p3IDCz | —/461c | 100/— | 2.69/2.50/0.19 | -5.41/-2.77/2.64 | 0.28/0.72 | |
| IDCz-DBS | 456/— | 99/— | 2.72/2.35/0.37 | —/—/— | —/— | [ |
| Compound | λem/nm (Sol/film) | ФPL/% (Sol/film) | ES1/ET1/∆EST/eV | HOMO/LUMO/Eg/eV | Td/Tg/℃ | Ref. |
|---|---|---|---|---|---|---|
| tDIDCz | 393/— | 60.0/— | 3.15/2.71/0.44 | -5.98/-2.59/3.39 | —/— | [ |
| m-FLDID | 400/404 | 71.0/— | 3.08/2.72/0.36 | -5.94/-2.62/3.32 | —/— | [ |
| m-FDIDCz-2DPA | 451a/458 | —/66.7 | —/—/— | -5.14/-2.39/2.75b | 545/— | [ |
| DICz | 446a/451 | 99/93 | —/—/— | —/—/— | —/— | [ |
| mMes2DICz | 445a/451 | 99/99 | —/—/— | -5.28/-2.77/2.51 | 455/— | |
| mtPh2DICz | 457a461 | 99/94 | —/—/— | -5.45/-2.69/3.02 | 496/— | |
| Cz-DICz | 457/460 | 98.6/98.4 | 2.71/2.37/0.34 | −5.55/−2.59/2.96/ | —/— | [ |
| DiICzMes4 | —/— | —/82 | 2.82/2.56/0.26 | -5.45/-2.43/3.02 | 450/— | [ |
| p-FLDID | 445/449 | 97.3/82.8 | 2.76/2.57/0.19 | -5.76/-2.90/2.85 | 0.32/0.65 | [ |
| BisICz | 436/442 | 81/76 | 2.84/2.55/0.29 | -5.80/-3.00/2.80 | 0.81/— | [ |
| tBisICz | 436/442 | 95/77 | 2.84/2.55/0.29 | -5.70/-2.90/2.80 | 0.48/0.47 | |
| tPBisICz | 445/450 | 91/78 | 2.81/2.54/0.27 | -5.90/-3.20/2.70 | 0.35/0.56 | |
| pICz-PPO | 438/— | 42/— | 2.82/2.47/0.35 | -5.34/-2.54/2.80 | 514/— | [ |
| pICz-2PPO | 439/— | 42/— | 2.82/2.46/0.36 | -5.37/-2.57/2.80 | 552/— | |
| tBisICz-DPA | 444/— | 96/— | 2.79/2.52/0.28 | -5.65/-2.89/2.76 | 0.45/0.51 | [ |
| tBisICz-PhCz | 440/— | 91/— | 2.82/2.51/0.31 | -5.67/-2.89/2.78 | 0.45/0.46 | |
| pICz | 441a/— | 88/— | 2.81/2.52/0.29 | -5.29/-2.5/2.79 | —/— | [ |
| pICz-TPA | 447a/— | 90/— | 2.77/2.45/0.32 | -5.14/-2.39/2.75 | —/— | |
| DPA-bisICZ | 500/505 | 78/92 | 2.48/2.34/0.14 | -4.91/-2.43/2.48 | 0.78/0.14 | [ |
| tBuDPA-bisICZ | 508/515 | 82/93 | 2.44/2.29/0.15 | -4.82/-2.39/2.43 | 0.79/0.14 | |
| MeODPA-bisICZ | 523/532 | 83/90 | 2.35/2.19/0.16 | -4.68/-2.33/2.35 | 0.78/0.12 | |
| 2,5-tDPAtDIDCz | —/438c | 88/— | 2.83/2.65/0.18 | -5.55/-2.74/2.81 | 0.26/0.62 | [ |
| 1,6-tDPAtDIDCz | —/415c | 92/— | 2.99/2.58/0.41 | -5.54/-2.66/2.88 | 0.92/— | |
| tBu-TiTAT | 452a/454 | —/76 | 2.90/2.54/0.36 | -5.39/-2.64/2.75b | 468/— | [ |
| tBu-TTAT | 425a/430 | —/36 | 3.13/2.60/0.53 | -5.30/-2.55/2.75b | 409/— | |
| α-NAICZ | 598/— | 97/— | 2.07/1.69/0.38 | -4.69/-2.56/2.13 | 431/— | [ |
| α-EtNAICZ | 620/— | 90/— | 2.00/1.69/0.31 | -4.53/-2.48/2.05 | 412/— | |
| NBisICz | 445/457 | —/63 | 2.79/2.37/0.42 | -5.68/-2.92/2.76b | 525/— | [ |
| NBisICz-PCz | 445/454 | —/64 | 2.79/2.37/0.42 | -5.65/-2.90/2.75b | 541/— | |
| NBisICz-DPA | 451/460 | —/64 | 2.75/2.37/0.37 | -5.71/-2.98/2.73b | 520/— | |
| ββICZ | 502/— | 99/— | 2.47/1.94/0.53 | -5.35/-2.85/2.50 | 518/— | [ |
| αβICZ | 472/— | 56/— | 2.64/2.11/0.53 | -5.55/-2.82/2.73 | —/— | |
| ββCNICZ | 501/— | 95/— | 2.47/1.93/0.54 | -5.51/-3.00/2.51 | 550/— | |
| t-mp3ICz | 436c | 99/— | 2.84/2.54/0.30 | -5.63/-2.84/2.79 | 0.49/0.50 | [ |
| p-mp3ICz | 444c | 97/— | 2.80/2.54/0.26 | -5.63/-2.87/2.76 | 0.35/0.62 | |
| t3IDCz | —/459c | 92/— | 2.70/2.49/0.21 | -5.50/-2.85/2.65 | 0.28/0.64 | [ |
| p3IDCz | —/461c | 100/— | 2.69/2.50/0.19 | -5.41/-2.77/2.64 | 0.28/0.72 | |
| IDCz-DBS | 456/— | 99/— | 2.72/2.35/0.37 | —/—/— | —/— | [ |
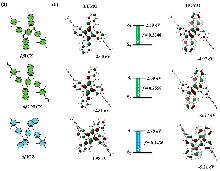


| Emitter | Doping conc./% | Von/V | CEmax/(cd•A−1) | EQEmax(100/1000)/% | ELmax/nm | CIE(x, y) | FWHM | Ref. |
|---|---|---|---|---|---|---|---|---|
| tDIDCz | 1 | 3.5 | — | 2.75 | 401 | (0.164, 0.018) | 14 | [ |
| 3 | 3.5 | — | 3.30 | 402 | (0.164, 0.019) | 20 | ||
| m-FLDID | 1 | 4.1 | — | 4.40 | 407 | (0.168, 0.024) | 17 | [ |
| 3 | 4.0 | — | 5.10 | 409 | (0.166, 0.025) | 22 | ||
| m-FDIDCz-2DPA | 2.64 | — | 8.32 (—/7.20) | 459 | (0.140, 0.105) | 30 | [ | |
| DICz | 1 | 3.8 | — | 5.5 (4.6/—) | — | (0.14, 0.10) | 35 | [ |
| mMes2DICz | 1 | 3.3 | — | 9.5 (7.8/—) | — | (0.15, 0.04) | 13 | |
| 2 | 3.1 | — | 10.2 (8.2/—) | — | (0.14, 0.05) | 15 | ||
| 4 | 3.0 | — | 10.6 (8.6/—) | — | (0.14, 0.09) | 33 | ||
| mtPh2DICz | 1 | 3.4 | — | 10.3 (8.3/—) | — | (0.13, 0.14) | 33 | |
| Cz-DICz (non- sensitized) | 1 | 3.1 | 8.59 | 8.79 | 460 | (0.14, 0.11) | 18 | [ |
| 2 | 3.1 | 7.77 | 7.93 | 461 | (0.13, 0.11) | 19 | ||
| 4 | 3.2 | 7.28 | 7.65 | 462 | (0.13, 0.12) | 19 | ||
| Cz-DICza | 1 | 2.9 | 25.62 | 25.6 | 460 | (0.14, 0.10) | 18 | |
| 2 | 2.8 | 25.28 | 23.8 | 460 | (0.14, 0.11) | 18 | ||
| 4 | 2.8 | 25.21 | 22.1 | 461 | (0.14, 0.11) | 18 | ||
| Cz-DICzb | — | 2.5 | 8.6 | 9.4 | 460 | (0.13, 0.09) | 15 | |
| DiICzMes4 | — | 5.2 | — | 3.0 (1.9/—) | 446 | (0.15, 0.11) | — | [ |
| p-FLDID | 1 | 3.5 | 11.2 | 18.7 | 452 | (0.147, 0.058) | 23 | [ |
| BisICz | 1 | — | 2.9 | 6.5 | 437 | (0.16, 0.04) | 24 | [ |
| tBisICz | 1 | — | 8.4 | 15.1 | 445 | (0.16, 0.05) | 22 | |
| tPBisICz | 1 | — | 13.5 | 23.1 | 452 | (0.15, 0.05) | 21 | |
| pICz-PPOa | 1 | 3.6 | 8.7 | 12.1 (7.1/—) | 442 | (0.16, 0.08) | 20 | [ |
| pICz-2PPOa | 1 | 3.6 | 12.6 | 17.7 (12.8/—) | 441 | (0.16, 0.07) | 19 | |
| tBisICz-DPA | 1 | — | 11.0 | 20.0 | 448 | (0.15, 0.05) | 28 | [ |
| 3 | — | 12.3 | 20.4 | 452 | (0.15, 0.05) | 28 | ||
| 5 | — | 12.1 | 19.5 | 453 | (0.15, 0.06) | 29 | ||
| tBisICz-PhCz | 1 | — | 8.5 | 19.7 | 445 | (0.17, 0.05) | 20 | |
| 3 | — | 11.6 | 24.9 | 446 | (0.16, 0.04) | 19 | ||
| 5 | — | 7.7 | 16.6 | 447 | (0.15, 0.04) | 19 | ||
| pICzc | 3.5 | 30.1 | 32.0 (6.7/—) | 445 | (0.15, 0.10) | — | [ | |
| pICz-TPAc | 3.6 | 31.5 | 34.7 (11.2/—) | 445 | (0.15, 0.085) | — | ||
| DPA-bisICZ | 2.5 | — | 24.4 | 508 | (0.17, 0.66) | 34 | [ | |
| tBuDPA-bisICZ | 2.9 | — | 24.7 | 514 | (0.20, 0.68) | 35 | ||
| MeODPA-bisICZ | 2.1 | — | 22.6 | 532 | (0.31, 0.65) | 40 | ||
| 2,5-tDPAtDIDCzd | 1 | 3.7 (@1.0 cd•m-2) | 24.2 | 23.4 | 464 | (0.13, 0.12) | 36 | [ |
| 2,5-tDPAtDIDCze | 1 | 4.1 (@1.0 cd•m-2) | 34.1 | 30.8 | 466 | (0.13, 0.14) | 38 | |
| 1,6-tDPAtDIDCzd | 1 | 3.5 (@1.0 cd•m-2) | 3.4 | 6.4 | 437 | (0.16, 0.04) | 32 | |
| 1,6-tDPAtDIDCze | 1 | 3.9 (@1.0 cd•m-2) | 8.7 | 7.3 | 438 | (0.16, 0.05) | 36 | |
| tBu-TiTAT | 6.0 | 7.8 | 6.3 (4.1/2.6) | 454 | (0.14, 0.14) | [ | ||
| tBu-TiTATc | 6.0 | 19.6 | 12.6 (7.1/5.7) | 454 | (0.16, 0.19) | 55 | ||
| α-NAICZ | — | 2.3 | 27.4 | 20.2 (—/19.6), 18.6f | 610 | (0.65, 0.35) | 38 | [ |
| α-EtNAICZ | — | 2.2 | 5.7 | 6.3 (—/4.5), 3.9f | 631 | (0.51, 0.39) | 44 | |
| NBisICz | 5% TTF | 6.1 | 7.9 | 7.1 (—/6.9) | 469 | (0.127, 0.148) | 48.4 | [ |
| NBisICz-PCz | 2% PSF | 7.2 | 16.6 | 18.8 (—/5.5) | 453 | (0.143, 0.076) | 22 | |
| NBisICz-DPA | 2% PSF | 6.6 | 19.1 | 20.6 (—/7.9) | 454 | (0.141, 0.085) | 27.5 | |
| ββICZa | 1 | 2.4 | 100.9 | 30.9 (—/28.9), 23.2f | 508 | (0.22, 0.64) | 19 | [ |
| ββCNICZa | 1 | 2.6 | 87.3 | 27.0 (—/25.5), 18.6f | 509 | (0.21, 0.65) | 19 | |
| t-mp3ICz | 1 | Vd-5.7 | 12.2 | 24.3 | 446 | (0.154, 0.044) | 21 | [ |
| p-mp3ICz | 1 | Vd-5.7 | 16.1 | 24.2 | 451 | (0.147, 0.061) | 23 | |
| 1 | Vd-5.3 | 18.8 | 26.8 | 452 | (0.142, 0.061) | 25 | ||
| t3IDCz | 1 | 6.9 (@1000 cd•m-2) | 36.9 | 30.0 | 472 | (0.119, 0.161) | 25 | [ |
| t3IDCg | 1 | 6.9 (@100 cd•m-2) | 35.4 | 30.9 | 471 | (0.116, 0.158) | 26 | |
| p3IDCz | 1 | 6.9 (@1000 cd•m-2) | 37.9 | 30.9 | 472 | (0.120, 0.158) | 23 | |
| p3IDCzg | 1 | 6.9 (@100 cd•m-2) | 43.4 | 33.8 | 473 | (0.111, 0.188) | 28 | |
| p3IDCzh | 1 | 6.9 (@100 cd•m-2) | 42.6 | 30.5 | 474 | (0.116, 0.213) | 33 | |
| IDCz-DBS | — | — | — | 31.1 | 461 | (0.135, 0.160) | 21 | [ |
| Emitter | Doping conc./% | Von/V | CEmax/(cd•A−1) | EQEmax(100/1000)/% | ELmax/nm | CIE(x, y) | FWHM | Ref. |
|---|---|---|---|---|---|---|---|---|
| tDIDCz | 1 | 3.5 | — | 2.75 | 401 | (0.164, 0.018) | 14 | [ |
| 3 | 3.5 | — | 3.30 | 402 | (0.164, 0.019) | 20 | ||
| m-FLDID | 1 | 4.1 | — | 4.40 | 407 | (0.168, 0.024) | 17 | [ |
| 3 | 4.0 | — | 5.10 | 409 | (0.166, 0.025) | 22 | ||
| m-FDIDCz-2DPA | 2.64 | — | 8.32 (—/7.20) | 459 | (0.140, 0.105) | 30 | [ | |
| DICz | 1 | 3.8 | — | 5.5 (4.6/—) | — | (0.14, 0.10) | 35 | [ |
| mMes2DICz | 1 | 3.3 | — | 9.5 (7.8/—) | — | (0.15, 0.04) | 13 | |
| 2 | 3.1 | — | 10.2 (8.2/—) | — | (0.14, 0.05) | 15 | ||
| 4 | 3.0 | — | 10.6 (8.6/—) | — | (0.14, 0.09) | 33 | ||
| mtPh2DICz | 1 | 3.4 | — | 10.3 (8.3/—) | — | (0.13, 0.14) | 33 | |
| Cz-DICz (non- sensitized) | 1 | 3.1 | 8.59 | 8.79 | 460 | (0.14, 0.11) | 18 | [ |
| 2 | 3.1 | 7.77 | 7.93 | 461 | (0.13, 0.11) | 19 | ||
| 4 | 3.2 | 7.28 | 7.65 | 462 | (0.13, 0.12) | 19 | ||
| Cz-DICza | 1 | 2.9 | 25.62 | 25.6 | 460 | (0.14, 0.10) | 18 | |
| 2 | 2.8 | 25.28 | 23.8 | 460 | (0.14, 0.11) | 18 | ||
| 4 | 2.8 | 25.21 | 22.1 | 461 | (0.14, 0.11) | 18 | ||
| Cz-DICzb | — | 2.5 | 8.6 | 9.4 | 460 | (0.13, 0.09) | 15 | |
| DiICzMes4 | — | 5.2 | — | 3.0 (1.9/—) | 446 | (0.15, 0.11) | — | [ |
| p-FLDID | 1 | 3.5 | 11.2 | 18.7 | 452 | (0.147, 0.058) | 23 | [ |
| BisICz | 1 | — | 2.9 | 6.5 | 437 | (0.16, 0.04) | 24 | [ |
| tBisICz | 1 | — | 8.4 | 15.1 | 445 | (0.16, 0.05) | 22 | |
| tPBisICz | 1 | — | 13.5 | 23.1 | 452 | (0.15, 0.05) | 21 | |
| pICz-PPOa | 1 | 3.6 | 8.7 | 12.1 (7.1/—) | 442 | (0.16, 0.08) | 20 | [ |
| pICz-2PPOa | 1 | 3.6 | 12.6 | 17.7 (12.8/—) | 441 | (0.16, 0.07) | 19 | |
| tBisICz-DPA | 1 | — | 11.0 | 20.0 | 448 | (0.15, 0.05) | 28 | [ |
| 3 | — | 12.3 | 20.4 | 452 | (0.15, 0.05) | 28 | ||
| 5 | — | 12.1 | 19.5 | 453 | (0.15, 0.06) | 29 | ||
| tBisICz-PhCz | 1 | — | 8.5 | 19.7 | 445 | (0.17, 0.05) | 20 | |
| 3 | — | 11.6 | 24.9 | 446 | (0.16, 0.04) | 19 | ||
| 5 | — | 7.7 | 16.6 | 447 | (0.15, 0.04) | 19 | ||
| pICzc | 3.5 | 30.1 | 32.0 (6.7/—) | 445 | (0.15, 0.10) | — | [ | |
| pICz-TPAc | 3.6 | 31.5 | 34.7 (11.2/—) | 445 | (0.15, 0.085) | — | ||
| DPA-bisICZ | 2.5 | — | 24.4 | 508 | (0.17, 0.66) | 34 | [ | |
| tBuDPA-bisICZ | 2.9 | — | 24.7 | 514 | (0.20, 0.68) | 35 | ||
| MeODPA-bisICZ | 2.1 | — | 22.6 | 532 | (0.31, 0.65) | 40 | ||
| 2,5-tDPAtDIDCzd | 1 | 3.7 (@1.0 cd•m-2) | 24.2 | 23.4 | 464 | (0.13, 0.12) | 36 | [ |
| 2,5-tDPAtDIDCze | 1 | 4.1 (@1.0 cd•m-2) | 34.1 | 30.8 | 466 | (0.13, 0.14) | 38 | |
| 1,6-tDPAtDIDCzd | 1 | 3.5 (@1.0 cd•m-2) | 3.4 | 6.4 | 437 | (0.16, 0.04) | 32 | |
| 1,6-tDPAtDIDCze | 1 | 3.9 (@1.0 cd•m-2) | 8.7 | 7.3 | 438 | (0.16, 0.05) | 36 | |
| tBu-TiTAT | 6.0 | 7.8 | 6.3 (4.1/2.6) | 454 | (0.14, 0.14) | [ | ||
| tBu-TiTATc | 6.0 | 19.6 | 12.6 (7.1/5.7) | 454 | (0.16, 0.19) | 55 | ||
| α-NAICZ | — | 2.3 | 27.4 | 20.2 (—/19.6), 18.6f | 610 | (0.65, 0.35) | 38 | [ |
| α-EtNAICZ | — | 2.2 | 5.7 | 6.3 (—/4.5), 3.9f | 631 | (0.51, 0.39) | 44 | |
| NBisICz | 5% TTF | 6.1 | 7.9 | 7.1 (—/6.9) | 469 | (0.127, 0.148) | 48.4 | [ |
| NBisICz-PCz | 2% PSF | 7.2 | 16.6 | 18.8 (—/5.5) | 453 | (0.143, 0.076) | 22 | |
| NBisICz-DPA | 2% PSF | 6.6 | 19.1 | 20.6 (—/7.9) | 454 | (0.141, 0.085) | 27.5 | |
| ββICZa | 1 | 2.4 | 100.9 | 30.9 (—/28.9), 23.2f | 508 | (0.22, 0.64) | 19 | [ |
| ββCNICZa | 1 | 2.6 | 87.3 | 27.0 (—/25.5), 18.6f | 509 | (0.21, 0.65) | 19 | |
| t-mp3ICz | 1 | Vd-5.7 | 12.2 | 24.3 | 446 | (0.154, 0.044) | 21 | [ |
| p-mp3ICz | 1 | Vd-5.7 | 16.1 | 24.2 | 451 | (0.147, 0.061) | 23 | |
| 1 | Vd-5.3 | 18.8 | 26.8 | 452 | (0.142, 0.061) | 25 | ||
| t3IDCz | 1 | 6.9 (@1000 cd•m-2) | 36.9 | 30.0 | 472 | (0.119, 0.161) | 25 | [ |
| t3IDCg | 1 | 6.9 (@100 cd•m-2) | 35.4 | 30.9 | 471 | (0.116, 0.158) | 26 | |
| p3IDCz | 1 | 6.9 (@1000 cd•m-2) | 37.9 | 30.9 | 472 | (0.120, 0.158) | 23 | |
| p3IDCzg | 1 | 6.9 (@100 cd•m-2) | 43.4 | 33.8 | 473 | (0.111, 0.188) | 28 | |
| p3IDCzh | 1 | 6.9 (@100 cd•m-2) | 42.6 | 30.5 | 474 | (0.116, 0.213) | 33 | |
| IDCz-DBS | — | — | — | 31.1 | 461 | (0.135, 0.160) | 21 | [ |
| [1] |
doi: 10.1063/1.98799 |
| [2] |
doi: 10.1039/c2tc00584k |
| [3] |
doi: 10.1038/nature07423 |
| [4] |
doi: 10.1038/25954 |
| [5] |
doi: 10.1038/nature11687 |
| [6] |
|
| [7] |
pmid: 17802593 |
| [8] |
doi: 10.1002/adma.v28.14 |
| [9] |
doi: 10.1002/anie.v54.46 |
| [10] |
doi: 10.1007/s11426-020-9944-1 |
| [11] |
doi: 10.1002/adom.v9.4 |
| [12] |
doi: 10.1002/smll.v16.14 |
| [13] |
doi: 10.1038/s41427-021-00318-8 |
| [14] |
doi: 10.1002/adom.v10.22 |
| [15] |
doi: 10.6023/A23040186 |
|
(王一诺, 邵世祥, 王利祥, 化学学报, 2023, 81, 1202.)
doi: 10.6023/A23040186 |
|
| [16] |
doi: 10.1002/advs.v8.20 |
| [17] |
doi: 10.1039/D2TC00921H |
| [18] |
doi: 10.1039/c0nj00100g |
| [19] |
doi: 10.1016/j.tet.2013.02.007 |
| [20] |
|
| [21] |
doi: 10.1021/jacs.0c10081 |
| [22] |
doi: 10.1002/anie.v58.47 |
| [23] |
doi: 10.31635/ccschem.021.202101033 |
| [24] |
doi: 10.1002/adma.v32.40 |
| [25] |
doi: 10.1002/adom.v7.7 |
| [26] |
doi: 10.1039/D1MH01383A |
| [33] |
doi: 10.1002/sdtp.v54.1 |
| [34] |
doi: 10.1007/s11426-018-9413-5 |
| [35] |
doi: 10.1039/D3TC03808D |
| [36] |
doi: 10.1016/j.dyepig.2021.109580 |
| [37] |
doi: 10.1016/j.orgel.2018.06.031 |
| [38] |
doi: 10.1021/acs.chemmater.6b01691 |
| [39] |
doi: 10.1039/c3cc48531e |
| [40] |
|
| [41] |
doi: 10.1063/1.2960348 |
| [42] |
|
| [43] |
doi: 10.1002/advs.v10.26 |
| [44] |
doi: 10.1002/adma.v31.21 |
| [45] |
doi: 10.1002/anie.v60.22 |
| [46] |
|
| [47] |
doi: 10.1038/s41467-023-40481-w pmid: 37558680 |
| [48] |
doi: 10.1002/adom.v11.2 |
| [49] |
doi: 10.1016/j.cej.2023.143423 |
| [50] |
doi: 10.1038/s41566-019-0476-5 |
| [51] |
doi: 10.1038/s41467-019-08495-5 pmid: 30723203 |
| [52] |
doi: 10.1021/jacs.1c09058 |
| [53] |
|
| [54] |
doi: 10.1038/srep08429 pmid: 25673259 |
| [55] |
doi: 10.1002/adom.v9.13 |
| [56] |
doi: 10.1002/advs.v11.40 |
| [57] |
doi: 10.1021/jacs.7b10578 pmid: 29120174 |
| [58] |
doi: 10.1002/adma.v35.22 |
| [59] |
doi: 10.1002/aelm.v8.3 |
| [60] |
doi: 10.1073/pnas.092143399 |
| [61] |
doi: 10.1016/j.mattod.2023.09.002 |
| [62] |
doi: 10.1038/s41566-017-0087-y |
| [63] |
doi: 10.1021/jacsau.1c00179 |
| [64] |
doi: 10.1002/adma.v34.33 |
| [27] |
doi: 10.1021/acsami.1c02635 |
| [28] |
doi: 10.1016/j.cej.2020.125125 |
| [29] |
doi: 10.1039/C9TC06434F |
| [30] |
|
| [31] |
doi: 10.1002/advs.v11.11 |
| [32] |
doi: 10.1021/acsami.7b09351 |
| [65] |
doi: 10.1002/adma.v34.9 |
| [66] |
doi: 10.1002/adma.v36.45 |
| [67] |
doi: 10.1002/adom.v12.3 |
| [1] | Xiaomei Jiang, Haijun Tan, Liwen Ping, Zhenguang Hu. Benzimidazole Acceptor (A)-π-Donor (D) Type Small Molecule Blue Purple Traditional Fluorescent Material [J]. Chinese Journal of Organic Chemistry, 2025, 45(8): 2896-2906. |
| [2] | Jiajie Wu, Chen Zhu, Yipu Wang, Yongzhen Yang, Haoke Zhang. Through-Space Conjugation in Multiarylalkanes [J]. Chinese Journal of Organic Chemistry, 2025, 45(11): 4037-4047. |
| [3] | Jianrong Wu, Min Song, Fuming Liu, Dongying Zhou, Liangsheng Liao, Zuoquan Jiang. Bifunctional Modification Strategy for Constructing Boron-Based Multi-resonance Thermally Activated Delayed Fluorescence Materials with Blue Emission [J]. Chinese Journal of Organic Chemistry, 2025, 45(11): 4152-4162. |
| [4] | Yang Xia, Kai-Yin Ren, Xiao-Ye Wang. Recent Advances in Narrowband Organic Afterglow Materials [J]. Chinese Journal of Organic Chemistry, 2025, 45(11): 4026-4036. |
| [5] | Nan Li, Yunsheng Wang, Zhen Li. Photoinduced Room-Temperature Phosphorescence of Triphenylamine-Phenothiazine Derivative-Doped Polymers [J]. Chinese Journal of Organic Chemistry, 2024, 44(8): 2487-2494. |
| [6] | Junchu He, Junqi Wu, Jianghui Wang, Jingwen Xu, BenZhong Tang, Zujin Zhao. Blue Aggregation-Induced Delayed Fluorescence Materials with 5,10-Dihydrodibenzo[b,e][1,4]azasiline as Donor [J]. Chinese Journal of Organic Chemistry, 2024, 44(8): 2513-2522. |
| [7] | Yongqing Li, Yuqing Peng, Yucai Cao, Guiping Cao. Synthesis of 2,7-Diaryl Substituted Fluorenes and Fluorenones [J]. Chinese Journal of Organic Chemistry, 2024, 44(5): 1494-1505. |
| [8] | Jing Sun, Zhijie Fan, Jikuan Du, Shuo Li, Yanqin Miao, Bo Zhao, Hailiang Dong, Hua Wang. Design and Synthesis of a Y-Type Thioxanthone-Carbazole for the Application in Blue and Yellow Organic Light-Emitting Diodes [J]. Chinese Journal of Organic Chemistry, 2024, 44(4): 1210-1217. |
| [9] | Chongyang Zeng, Ping Hu, Biqin Wang, Wenyan Fang, Keqing Zhao. Cyanostilbene Bridged Triphenylene Dyad Stimuli-Responsive Discotic Liquid Crystal: Synthesis, Properties and Applications [J]. Chinese Journal of Organic Chemistry, 2023, 43(9): 3287-3296. |
| [10] | Yi Zhang, Cheng-Zhuo Du, Ji-Kun Li, Xiao-Ye Wang. Recent Advances in Multi-Resonance Thermally Activated Delayed Fluorescence Materials Based on B,N-Heteroarenes [J]. Chinese Journal of Organic Chemistry, 2023, 43(5): 1645-1690. |
| [11] | Xiaodong Yang, Xiaokang Zheng, Hailiang Dong, Jing Sun, Hua Wang. Research Progress of Circularly Polarized Thermally Activated Delayed Fluorescence Materials and Devices [J]. Chinese Journal of Organic Chemistry, 2023, 43(4): 1292-1309. |
| [12] | Huiming Lu, Lamaocao Ma, Hengchang Ma. Research Progress and Prospect of Aggregation-Induced Emission Supramolecular Luminescence Materials [J]. Chinese Journal of Organic Chemistry, 2023, 43(12): 4075-4105. |
| [13] | Xiaoyang Xu, Meiyan Liu, Chenglong Li, Xiaoming Wu, Xuguang Liu. Recent Advance of 1,4-BN Heteroaromatics in China [J]. Chinese Journal of Organic Chemistry, 2023, 43(11): 3826-3843. |
| [14] | Qin Chengyuan, Liu Wei, Nie Yong, Gao Ying, Miao Jinling, Li Tianrui, Jiang Xuchuan. Advances in Organofluorine Compounds with Aggregation-Induced Emission [J]. Chinese Journal of Organic Chemistry, 2020, 40(8): 2232-2253. |
| [15] | Yan Zi'ang, Zou Lei, Ma Xiang. Recent Advances in Pure Organic Luminescent Supramolecular Materials [J]. Chinese Journal of Organic Chemistry, 2020, 40(7): 1814-1822. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||