Chinese Journal of Organic Chemistry ›› 2025, Vol. 45 ›› Issue (11): 3998-4012.DOI: 10.6023/cjoc202503036 Previous Articles Next Articles
REVIEWS
收稿日期:2025-03-31
修回日期:2025-06-02
发布日期:2025-06-30
基金资助:Received:2025-03-31
Revised:2025-06-02
Published:2025-06-30
Contact:
*E-mail: zhaozheng@cuhk.edu.cn
Supported by:Share
Yixuan Chen, Zheng Zhao. Aggregation-Induced Emission Photosensitizers for Photodynamic Antimicrobial Therapy[J]. Chinese Journal of Organic Chemistry, 2025, 45(11): 3998-4012.








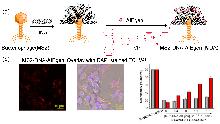

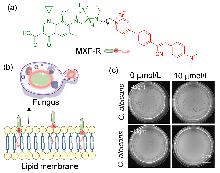

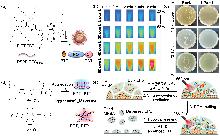

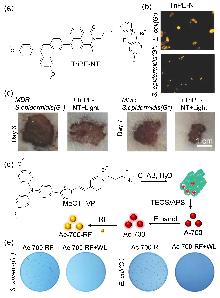




| [1] |
doi: 10.1002/adma.v37.23 |
| [2] |
doi: 10.1002/adma.v32.18 |
| [3] |
doi: 10.1039/C7CS00807D |
| [4] |
doi: 10.1038/s41551-017-0187-5 pmid: 29955439 |
| [68] |
doi: 10.1016/j.carbpol.2023.121186 |
| [69] |
doi: 10.1039/C5CC03807C |
| [70] |
(a)
|
|
(b)
|
|
| [71] |
doi: 10.1002/adhm.v13.30 |
| [72] |
|
| [5] |
doi: 10.1038/s41586-022-05019-y |
| [6] |
(a)
doi: 10.3201/eid2111.150452 |
|
(b)
doi: 10.1016/S0140-6736(21)02724-0 |
|
| [7] |
doi: 10.1039/D1CS00647A |
| [8] |
doi: 10.1002/adma.v30.36 |
| [73] |
doi: 10.1016/j.jcis.2019.03.052 |
| [74] |
doi: 10.1002/adfm.v28.42 |
| [75] |
doi: 10.1002/adfm.v32.23 |
| [76] |
doi: 10.1002/adma.v35.6 |
| [77] |
doi: 10.1021/acsnano.0c05330 |
| [78] |
doi: 10.1038/s41598-023-41315-x |
| [79] |
doi: 10.1016/j.foodhyd.2021.107147 |
| [80] |
doi: 10.1016/j.cej.2024.150675 |
| [81] |
doi: 10.1016/j.biomaterials.2021.121007 |
| [82] |
|
| [9] |
doi: 10.7150/thno.31844 |
| [10] |
(a)
|
|
(b)
doi: 10.1016/j.cej.2024.153951 |
|
| [11] |
(a)
doi: 10.1016/j.cclet.2023.108312 |
|
(b)
|
|
| [12] |
doi: 10.1002/adma.v33.48 |
| [13] |
|
| [14] |
|
| [15] |
doi: 10.1002/anie.v59.25 |
| [16] |
doi: 10.1021/acs.chemrev.5b00263 pmid: 26492387 |
| [17] |
doi: 10.1038/s41467-019-10818-5 pmid: 31273202 |
| [18] |
(a)
doi: 10.1002/adfm.v30.39 |
|
(b)
|
|
| [19] |
(a)
|
|
(b)
doi: 10.1002/adfm.v34.38 |
|
|
(c)
|
|
| [20] |
(a)
doi: 10.1073/pnas.1708556114 |
|
(b)
doi: 10.1021/acssuschemeng.8b03540 |
|
|
(c)
doi: 10.1002/smll.v20.28 |
|
| [21] |
doi: 10.1039/C7QM00125H |
| [22] |
doi: 10.1021/acsami.8b07516 |
| [23] |
doi: 10.3389/fchem.2022.1088935 |
| [24] |
doi: 10.1021/jacs.9b07162 |
| [25] |
doi: 10.1039/C9TC04427B |
| [26] |
doi: 10.1002/adfm.v31.36 |
| [27] |
(a)
doi: 10.1016/j.biomaterials.2019.119582 |
|
(b)
doi: 10.1016/j.biomaterials.2020.120340 |
|
| [28] |
doi: 10.1021/acsnano.3c04266 |
| [29] |
doi: 10.1002/smtd.v4.7 |
| [30] |
(a)
doi: 10.1021/acsami.2c18835 |
|
(b)
|
|
| [31] |
(a)
doi: 10.1021/acsnano.2c05821 |
|
(b)
doi: 10.1021/jacsau.4c00915 |
|
| [32] |
doi: 10.1021/acsnano.2c01721 pmid: 35917549 |
| [33] |
doi: 10.1016/j.ccr.2018.03.020 |
| [34] |
|
| [35] |
Umesh; Chandran, V.; Saha, P.; Nath, D.; Bera, S.; Bhattacharya, S.; Pal, A. Nanoscale 2024, 16, 13050.
doi: 10.1039/D4NR01011F |
| [36] |
|
| [37] |
doi: 10.1021/acsabm.0c01206 |
| [83] |
doi: 10.1039/D1SC00045D |
| [84] |
|
| [85] |
doi: 10.1021/cbmi.5c00016 |
| [38] |
doi: 10.1016/j.dyepig.2022.110231 |
| [39] |
doi: 10.1016/j.cej.2023.147402 |
| [40] |
doi: 10.1021/acsami.0c11161 |
| [41] |
doi: 10.1021/acs.inorgchem.4c05064 |
| [42] |
|
| [43] |
(a)
doi: 10.1016/j.biomaterials.2022.121695 |
|
(b)
doi: 10.1039/D4NR01769B |
|
|
(c)
|
|
| [44] |
doi: 10.1039/D2NR01550A |
| [45] |
(a)
doi: 10.1016/j.cej.2024.152216 pmid: 39902652 |
|
(b)
doi: 10.1039/d4nr04718d pmid: 39902652 |
|
| [46] |
|
| [47] |
doi: 10.1002/adfm.v33.45 |
| [48] |
(a)
doi: 10.1039/D3TB01240A |
|
(b)
|
|
| [49] |
(a)
doi: 10.1021/acsbiomaterials.4c00056 pmid: 39423317 |
|
(b)
doi: 10.1002/pen.v63.8 pmid: 39423317 |
|
|
(c)
pmid: 39423317 |
|
|
(d)
pmid: 39423317 |
|
|
(e)
pmid: 39423317 |
|
|
(f)
doi: 10.1021/acsnano.4c10452 pmid: 39423317 |
|
| [50] |
|
| [51] |
doi: 10.1039/D0TB02272A |
| [52] |
doi: 10.1002/adfm.v35.26 |
| [53] |
doi: 10.1002/adhm.v11.17 |
| [54] |
doi: 10.1016/j.immuni.2021.12.002 |
| [55] |
doi: S1525-0016(17)30113-2 pmid: 28391960 |
| [56] |
doi: 10.1002/anie.v59.24 |
| [57] |
(a)
|
|
(b)
|
|
|
(c)
|
|
| [58] |
doi: 10.1002/adfm.v30.31 |
| [59] |
|
| [60] |
(a)
doi: 10.1021/acsnano.2c01206 |
|
(b)
doi: 10.1002/adfm.v30.31 |
|
| [61] |
doi: 10.1021/acsnano.3c00796 |
| [62] |
|
| [63] |
doi: 10.1038/s41467-024-55060-w |
| [64] |
(a)
doi: 10.1038/s41586-025-08629-4 |
|
(b)
doi: 10.1002/anie.v58.45 |
|
| [65] |
doi: 10.1021/acsnano.3c09695 pmid: 38227824 |
| [66] |
(a)
doi: 10.1021/jp509697n |
|
(b)
|
|
|
(c)
doi: 10.1021/jp509697n |
|
|
(d)
doi: 10.1039/D0SC00256A |
|
|
(e)
doi: 10.1039/C9QM00732F |
|
|
(f)
doi: 10.1016/j.biomaterials.2021.120725 |
|
| [67] |
|
| [1] | Bingyan Chen, Jie Sun, Linghong Xiong, Xuewen He. Fluorescence Light-Up Detection and Imaging of Atypical Nucleic Acid Structures [J]. Chinese Journal of Organic Chemistry, 2025, 45(11): 4082-4107. |
| [2] | Xue Bai, Yili Xie, Junyuan Li, Zihua Mo, Qing Wan. Design of High Efficiency Cationic Photosensitizer and Its Application in Low Irradiation Dose Photodynamic Antibacterial [J]. Chinese Journal of Organic Chemistry, 2025, 45(11): 4143-4151. |
| [3] | Jianye Yang, Pei Zhou, Qifei Shen, Peijuan Zhang, Yanzi Xu, Bingjie Zhao, Dongfeng Dang. Progress of Organic Radical Materials in Biomedical Applications [J]. Chinese Journal of Organic Chemistry, 2025, 45(11): 4013-4025. |
| [4] | Zixuan Dong, Junjun Su, Hongfei Pan, Xiangkui Ren, Zhijian Chen. Near-Infrared Aggregation-Induced Emission (AIE) Molecules Based on Coupling of Triarylamine-Modified Benzothiadiazole Units [J]. Chinese Journal of Organic Chemistry, 2025, 45(1): 205-211. |
| [5] | Yan Ou, Lin Lan, Zhengxiong Wang, Zhiming Wang, BenZhong Tang. Preparation of Aggregation-Induced Emission Nucleic Acid Probes and Study of Their Nucleic Acid Sensing Principles [J]. Chinese Journal of Organic Chemistry, 2024, 44(8): 2554-2562. |
| [6] | Yujie Yang, Wei Cao, Jikai Yu, Zhixia Zhang, Li Xu, Hua Wang. Synthesis of Donor-Acceptor (D-A) Typed Phenylcyclooctatetrathiophenes and Their Performances on Aggregation Induced Emission and High Pressure Luminescence [J]. Chinese Journal of Organic Chemistry, 2024, 44(8): 2495-2503. |
| [7] | Hanyu Jia, Yuewen Yu, Guangxue Feng, BenZhong Tang. Construction of Type I Aggregation-Induced Emission Photosensitizers for Photodynamic Therapy via Photoinduced Electron Transfer Mechanism [J]. Chinese Journal of Organic Chemistry, 2024, 44(8): 2530-2537. |
| [8] | Jidong Zhang, Yao Yang, Jie Zhang, Wei She. Detection of Zn(II) by Tetraphenylethyene Fluorescent Probe Based on Aggregation-Induced Emission (AIE)-Excited State Intramolecular Proton Transfer (ESIPT) Effect [J]. Chinese Journal of Organic Chemistry, 2024, 44(4): 1337-1342. |
| [9] | Chongyang Zeng, Ping Hu, Biqin Wang, Wenyan Fang, Keqing Zhao. Cyanostilbene Bridged Triphenylene Dyad Stimuli-Responsive Discotic Liquid Crystal: Synthesis, Properties and Applications [J]. Chinese Journal of Organic Chemistry, 2023, 43(9): 3287-3296. |
| [10] | Yang Zhao, Panpan Chen, Lizhi Han, Enju Wang. Aggregation-Induced Emission and Cell Imaging of Triphenylimidazole Derivatives [J]. Chinese Journal of Organic Chemistry, 2023, 43(7): 2454-2461. |
| [11] | Yuehua Zhang, Fei Nie, Lu Zhou, Xiaofeng Wang, Yuan Liu, Yanping Huo, Wencheng Chen, Zujin Zhao. Synthesis and Optoelectronic Studies of Thermally Activated Delayed Fluorescence Materials Based on Benzothiazolyl Ketones [J]. Chinese Journal of Organic Chemistry, 2023, 43(11): 3876-3887. |
| [12] | Meng Liu, Yanru Huang, Xiaofei Sun, Lijun Tang. An “Aggregation-Induced Emission+Excited-State Intramolecular Proton Transfer” Mechanisms-Based Benzothiazole Derived Fluorescent Probe and Its ClO– Recognition [J]. Chinese Journal of Organic Chemistry, 2023, 43(1): 345-351. |
| [13] | Jidong Zhang, Wanlin Yan, Wenqiang Hu, Dian Guo, Dalong Zhang, Xiaoxin Quan, Xianpan Bu, Siyu Chen. Design and Synthesis of a Zn2+ Fluorescent Probe Based on Aggregation Induced Luminescence Properties [J]. Chinese Journal of Organic Chemistry, 2023, 43(1): 326-331. |
| [14] | Yangyang Li, Xiaofei Sun, Xiaoling Hu, Yuanyuan Ren, Keli Zhong, Xiaomei Yan, Lijun Tang. Synthesis of Triphenylamine Derivative and Its Recognition for Hg2+ with “OFF-ON” Fluorescence Response Based on Aggregation-Induced Emission (AIE) Mechanism [J]. Chinese Journal of Organic Chemistry, 2023, 43(1): 320-325. |
| [15] | Ze Guo, Di Wu, Lili Wang, Zheng Duan. BF3•Et2O Promoted Dienone-Phenol Type Rearrangement to Synthesize Phosphepine with Aggregation Induced Luminescence (AIE) Effect [J]. Chinese Journal of Organic Chemistry, 2022, 42(8): 2481-2487. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
