Chinese Journal of Organic Chemistry ›› 2025, Vol. 45 ›› Issue (11): 4048-4069.DOI: 10.6023/cjoc202504012 Previous Articles Next Articles
REVIEWS
收稿日期:2025-04-10
修回日期:2025-05-29
发布日期:2025-07-11
基金资助:
Cuiping Sun, Yuting Xue, Pangkuan Chen*( )
)
Received:2025-04-10
Revised:2025-05-29
Published:2025-07-11
Contact:
*E-mail: pangkuan@bit.edu.cn
Supported by:Share
Cuiping Sun, Yuting Xue, Pangkuan Chen. Recent Advances in Heterohelicene-Based Circularly Polarized Luminescence Materials[J]. Chinese Journal of Organic Chemistry, 2025, 45(11): 4048-4069.


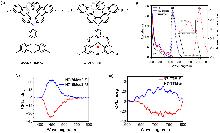

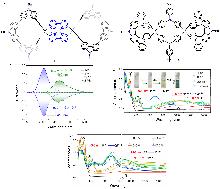

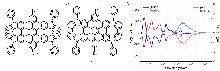

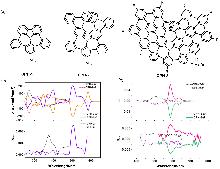





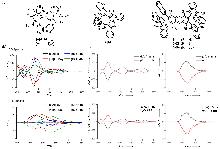





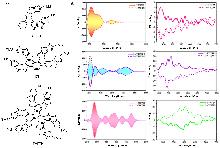

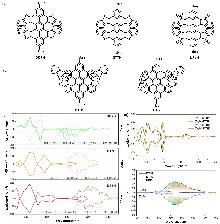

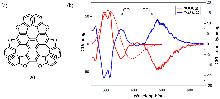

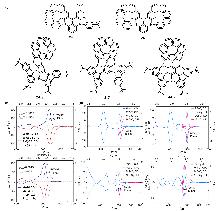

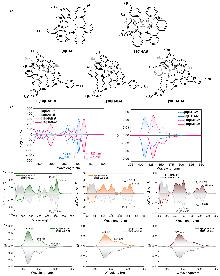

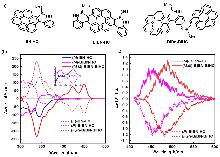

| Compound | ΦF | gabs/10-3 | glum/10-3 | Compound | ΦF | gabs/10-3 | glum/10-3 | |
|---|---|---|---|---|---|---|---|---|
| C | 0.86 | 0.48 | 0.22 | 19 | 0.48 | 7.5 | 0.59 | |
| rac-N7-Bmes2 | 0.32 | 4.17 | 3.39 | DO7H | 0.71 | 10 | 0.75 | |
| rac-N7-TTM | 0.5 | 2.7 | 3.57 | DT7H | 0.43 | 14 | 1.2 | |
| 1 | 0.26 | 33 | 1.5 | DSTH | 0.31 | 7.2 | 1.1 | |
| 2 | 0.99 | 14 | 5 | DTH1 | 0.83 | 4 | 2.2 | |
| 3 | 0.45 | — | 2.4 | DTH2 | 0.91 | 5.3 | 2.1 | |
| CPH-3 | 0.32 | 10 | 7 | 20 | 0.04 | 6.1 | 1.8 | |
| 5 | 0.47 | 3.5 | 0.94 | 21 | 0.53 | 4.6 | 0.5 | |
| 6a | 0.01 | 2.5 | — | 22 | 0.26 | 4 | 1.6 | |
| 6b | 0.12 | 1.9 | 1.6 | 23 | 0.1 | 3.6 | 2.1 | |
| 7 | 0.44 | 4.7 | 2.3 | A | 3.1 | — | 1.58 | |
| 8 | 0.32 | 3.9 | 1.9 | A' | 3.5 | — | 4.3 | |
| 9 | 0.61 | 2.1 | 0.74 | 2B | — | 1.6 | 0.47 | |
| 10 | 0.54 | 2.5 | 1.3 | 2B·F | — | 3 | 1.7 | |
| 11 | 0.1 | 4.7 | 3.5 | BN-HC | 0.11 | 0.6 | — | |
| 12 | 0.22 | 3.7 | 2.4 | BiBH-HC | 0.2 | 2.19 | 1.95 | |
| 13 | — | 2.8 | — | BiBH-BiHC | 0.87 | 2.15 | 2.06 | |
| 14 | — | — | — | 24 | 0.72 | 1.7 | 1.5 | |
| 9AH-Bu4 | 0.16 | 5.6 | 4.5 | 25 | 0.85 | 1.3 | 1.1 | |
| 11AH-Bu5 | 0.16 | 4.2 | 4.2 | 2-C1 | 0.85 | 1 | 0.75 | |
| 13AH-Bu6 | 0.09 | 4.2 | 1.7 | 2-C2 | 0.81 | 2.7 | 1.9 | |
| 15AH-Bu6 | 0.07 | 1.7 | 5.7 | 3-C2 | 0.59 | 4.1 | 2.3 | |
| 15 | 0.07 | — | — | BN[9]H | 0.98 | — | 5.8 | |
| 16-Et | 0.35 | 2.4 | 1 | [8]HAB | 0.31 | 36 | 24 | |
| 16-Bu | 0.35 | 2.3 | 1 | [10]HAB | 0.24 | 61 | 48 | |
| 16 | 0.4 | 2.98 | 4.3 | E[10]HAB-A | 0.82 | 24 | 17 | |
| 17 | 0.2 | — | 4.6 | E[10]HAB-B | 0.67 | 11 | 11 | |
| 18 | 3.5 | — | 12.5 | E[10]HAB-C | 0.72 | 12 | 8 | |
| TB[7]H | 0.19 | 3 | 15 | H1-M4 | 0.17 | 3.3 | 3.5 | |
| TS[7]H | — | 1 | — | SNSN-S | — | — | 1.2 |
| Compound | ΦF | gabs/10-3 | glum/10-3 | Compound | ΦF | gabs/10-3 | glum/10-3 | |
|---|---|---|---|---|---|---|---|---|
| C | 0.86 | 0.48 | 0.22 | 19 | 0.48 | 7.5 | 0.59 | |
| rac-N7-Bmes2 | 0.32 | 4.17 | 3.39 | DO7H | 0.71 | 10 | 0.75 | |
| rac-N7-TTM | 0.5 | 2.7 | 3.57 | DT7H | 0.43 | 14 | 1.2 | |
| 1 | 0.26 | 33 | 1.5 | DSTH | 0.31 | 7.2 | 1.1 | |
| 2 | 0.99 | 14 | 5 | DTH1 | 0.83 | 4 | 2.2 | |
| 3 | 0.45 | — | 2.4 | DTH2 | 0.91 | 5.3 | 2.1 | |
| CPH-3 | 0.32 | 10 | 7 | 20 | 0.04 | 6.1 | 1.8 | |
| 5 | 0.47 | 3.5 | 0.94 | 21 | 0.53 | 4.6 | 0.5 | |
| 6a | 0.01 | 2.5 | — | 22 | 0.26 | 4 | 1.6 | |
| 6b | 0.12 | 1.9 | 1.6 | 23 | 0.1 | 3.6 | 2.1 | |
| 7 | 0.44 | 4.7 | 2.3 | A | 3.1 | — | 1.58 | |
| 8 | 0.32 | 3.9 | 1.9 | A' | 3.5 | — | 4.3 | |
| 9 | 0.61 | 2.1 | 0.74 | 2B | — | 1.6 | 0.47 | |
| 10 | 0.54 | 2.5 | 1.3 | 2B·F | — | 3 | 1.7 | |
| 11 | 0.1 | 4.7 | 3.5 | BN-HC | 0.11 | 0.6 | — | |
| 12 | 0.22 | 3.7 | 2.4 | BiBH-HC | 0.2 | 2.19 | 1.95 | |
| 13 | — | 2.8 | — | BiBH-BiHC | 0.87 | 2.15 | 2.06 | |
| 14 | — | — | — | 24 | 0.72 | 1.7 | 1.5 | |
| 9AH-Bu4 | 0.16 | 5.6 | 4.5 | 25 | 0.85 | 1.3 | 1.1 | |
| 11AH-Bu5 | 0.16 | 4.2 | 4.2 | 2-C1 | 0.85 | 1 | 0.75 | |
| 13AH-Bu6 | 0.09 | 4.2 | 1.7 | 2-C2 | 0.81 | 2.7 | 1.9 | |
| 15AH-Bu6 | 0.07 | 1.7 | 5.7 | 3-C2 | 0.59 | 4.1 | 2.3 | |
| 15 | 0.07 | — | — | BN[9]H | 0.98 | — | 5.8 | |
| 16-Et | 0.35 | 2.4 | 1 | [8]HAB | 0.31 | 36 | 24 | |
| 16-Bu | 0.35 | 2.3 | 1 | [10]HAB | 0.24 | 61 | 48 | |
| 16 | 0.4 | 2.98 | 4.3 | E[10]HAB-A | 0.82 | 24 | 17 | |
| 17 | 0.2 | — | 4.6 | E[10]HAB-B | 0.67 | 11 | 11 | |
| 18 | 3.5 | — | 12.5 | E[10]HAB-C | 0.72 | 12 | 8 | |
| TB[7]H | 0.19 | 3 | 15 | H1-M4 | 0.17 | 3.3 | 3.5 | |
| TS[7]H | — | 1 | — | SNSN-S | — | — | 1.2 |
| [1] |
doi: 10.1021/cr00071a001 |
| [2] |
doi: 10.1002/chir.v35.4 |
| [3] |
pmid: 19881855 |
| [4] |
doi: 10.1039/C9CC03281A |
| [5] |
|
| [6] |
doi: 10.1021/jacs.1c10943 |
| [7] |
doi: 10.1021/jacs.2c03791 pmid: 35678629 |
| [8] |
|
| [9] |
doi: 10.1038/ncomms15066 |
| [10] |
doi: 10.1016/j.biomaterials.2013.08.013 |
| [11] |
doi: 10.1002/anie.v47:50 |
| [12] |
doi: 10.1021/ar000167x |
| [13] |
doi: 10.1021/jacs.1c10807 |
| [14] |
doi: 10.1021/acs.chemrev.6b00076 pmid: 27258218 |
| [15] |
doi: 10.1021/acs.chemrev.8b00637 |
| [16] |
doi: 10.1039/D2SC06000K |
| [17] |
doi: 10.1039/c2cs35154d pmid: 23151799 |
| [18] |
doi: 10.1021/acs.jpca.2c00432 |
| [19] |
|
| [20] |
|
| [21] |
doi: 10.1021/acs.inorgchem.3c02470 |
| [22] |
|
| [23] |
doi: 10.1021/jacs.8b06079 |
| [24] |
doi: 10.1016/j.chempr.2023.05.028 |
| [25] |
|
| [26] |
|
| [27] |
doi: 10.31635/ccschem.022.202101661 |
| [28] |
doi: 10.1039/d4sc01083c pmid: 38784742 |
| [29] |
doi: 10.1021/acs.orglett.2c02734 |
| [30] |
doi: 10.1021/jacs.4c03815 |
| [31] |
|
| [32] |
|
| [33] |
|
| [34] |
doi: 10.1021/jacs.7b10902 |
| [35] |
doi: 10.1021/jacs.8b09825 pmid: 30339380 |
| [36] |
doi: 10.1002/chem.202004720 pmid: 33205503 |
| [37] |
|
| [38] |
doi: 10.1021/cr200087r pmid: 22017405 |
| [39] |
doi: 10.1039/c2cs35111k pmid: 23151610 |
| [40] |
|
| [41] |
|
| [42] |
|
| [43] |
doi: 10.1007/s11426-023-1712-5 |
| [44] |
|
| [45] |
|
| [46] |
doi: 10.1039/D2TC02910C |
| [47] |
doi: 10.1039/D3SC05480B |
| [48] |
doi: 10.1021/acs.orglett.4c03849 |
| [49] |
|
| [50] |
doi: 10.1039/C6CC04674F |
| [51] |
doi: 10.1039/D1CC03314J |
| [52] |
doi: 10.6023/cjoc202100096 |
|
(刘豫健, 王朝晖, 有机化学, 2021, 41, 4844.)
doi: 10.6023/cjoc202100096 |
|
| [53] |
|
| [54] |
doi: 10.1002/adom.v13.6 |
| [55] |
|
| [56] |
|
| [57] |
doi: 10.1021/jacs.9b06240 pmid: 31322357 |
| [58] |
|
| [59] |
|
| [60] |
doi: 10.6023/cjoc202212038 |
|
(徐晓阳, 刘美艳, 李成龙, 刘旭光, 有机化学, 2023, 43, 1611.)
doi: 10.6023/cjoc202212038 |
|
| [61] |
doi: 10.6023/cjoc202304027 |
|
(徐晓阳, 刘美艳, 李成龙, 吴晓明, 刘旭光, 有机化学, 2023, 43, 3826.)
doi: 10.6023/cjoc202304027 |
|
| [62] |
|
| [63] |
doi: 10.1021/jacs.4c11404 |
| [64] |
doi: 10.1039/C6CS00368K |
| [65] |
|
| [66] |
|
|
(张祎, 杜呈卓, 李继坤, 王小野, 有机化学, 2023, 43, 1645.)
doi: 10.6023/cjoc202212037 |
|
| [67] |
doi: 10.1021/jacs.4c06997 |
| [68] |
|
| [69] |
doi: 10.1021/ja203206g |
| [70] |
doi: 10.1039/C4CC03944K |
| [71] |
doi: 10.1002/anie.v56.14 |
| [72] |
|
| [73] |
doi: 10.1021/acs.orglett.4c04821 pmid: 39973330 |
| [74] |
|
| [75] |
doi: 10.1039/D3TC00871A |
| [76] |
|
| [1] | Yanqiu Li, Yawei Jia, Pangkuan Chen. Recent Progress of Chiral Conjugated Organic Triarylboron (Ar3B) Luminescent Materials [J]. Chinese Journal of Organic Chemistry, 2025, 45(4): 1119-1136. |
| [2] | Qing-Yue Song, Guiyi Yang, Yue Zhang, Zijie Qiu. Research Progress on the Synthesis and Optoelectronic Properties of Helicene Derivatives [J]. Chinese Journal of Organic Chemistry, 2025, 45(11): 3953-3973. |
| [3] | Chenyang Zhao, Jingxiao Ren, Guanghui Ouyang, Minghua Liu. Supramolecular Assembly and Circularly Polarized Luminescence Regulation of a Chiral Dipyrene-Glutamic Acid Amphiphile [J]. Chinese Journal of Organic Chemistry, 2025, 45(11): 4171-4177. |
| [4] | Ziwei Chen, Sikang Duan, Yihui Wang, Huadan Fan, Mengjie Yang, Sisi Wang, Hua Lu. Peripheral-Group Induced Chirality of β-Isoindigo Based Aza Dipyrrometheneboron Difluoride (BODIPY) Analogs [J]. Chinese Journal of Organic Chemistry, 2025, 45(11): 4163-4170. |
| [5] | Yufei Xia, Li Jiang, Qiao Yang, Xiu Yu, Fengkun Chen. Triple Aza[6]helicenes with Circularly Polarized Luminescence: N-Alkylation as a Tool to Tune the Chiroptical Properties [J]. Chinese Journal of Organic Chemistry, 2024, 44(9): 2841-2846. |
| [6] | Lihua Wang, Xushun Gong, Ting Lei, Shizhi Jiang. Research Progress on Asymmetric Synthesis of Flavanones [J]. Chinese Journal of Organic Chemistry, 2022, 42(3): 758-769. |
| [7] | Jinguo Liu, Feng Yin, Jun Hu, Yong Ju. Fabrication and Applications of Supramolecular Chiral Assemblies [J]. Chinese Journal of Organic Chemistry, 2021, 41(3): 1031-1052. |
| [8] | Shi Zhan, Nie Kerui, Liu Chang, Zhang Mingzhi, Zhang Weihua. Biological Activities of 3-(5-Oxazolyl)indole Natural Products and Advances on Synthesis of Its Derivatives [J]. Chinese Journal of Organic Chemistry, 2020, 40(2): 327-338. |
| [9] | Xu Huanji, Li Zheming, Wu Yunqiu, Luo Di, Qiu Li, Xie Jizhao, Li Xuehua. Advances on Synthesis of Flavonoid Glycosides [J]. Chin. J. Org. Chem., 2019, 39(7): 1875-1890. |
| [10] | Hou Fangzhan, Mei Chong-Yu, Liang Long, Wang Hongyu, Xie Guanghui, Lu Zhengquan, Li Jingjing, Li Wei-Shi. Benzodithiophene-Cored Small Optoelectronic Molecules: Influence of Extension Direction of Conjugated Segments [J]. Chin. J. Org. Chem., 2016, 36(7): 1586-1595. |
| [11] | Pan Lingxiang, Luo Wenwen, Chen Ming, Liu Junkai, Xu Lu, Hu Rongrong, Zhao Zujin, Qin Anjun, Tang BenZhong. Tetraphenylpyrazine-Based Luminogens with Aggregation-Enhanced Emission Characteristics: Preparation and Property [J]. Chin. J. Org. Chem., 2016, 36(6): 1316-1324. |
| [12] | Zhang Li, Gao Shutao, Liu Weihua, Tang Ranxiao, Shang Ningzhao, Wang Chun, Wang Zhi. Research Progress of Graphene and Its Composites in Organic Synthesis [J]. Chin. J. Org. Chem., 2014, 34(8): 1542-1548. |
| [13] | Huang Shahua, Huang Mengyuan, Jia Xueshun, Hong Ran. Chemistry and Biology of Bakuchiol [J]. Chin. J. Org. Chem., 2014, 34(12): 2412-2423. |
| [14] | Wang Chun, Gao Shutao, Zhou Xin, Wu Qiuhua, Jiao Caina, Wang Zhi. Research Progress of Plasmonic Photocatalyst in Organic Synthesis [J]. Chin. J. Org. Chem., 2014, 34(11): 2217-2223. |
| [15] | Zheng Shuyan, Yu Chunhui, Shen Zhengwu. Progresses in the Total Synthesis of Huperzine A [J]. Chin. J. Org. Chem., 2013, 33(11): 2261-2270. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||