Chinese Journal of Organic Chemistry ›› 2026, Vol. 46 ›› Issue (1): 106-117.DOI: 10.6023/cjoc202506035 Previous Articles Next Articles
ARTICLES
洪赛†, 尹发红†, 陈明慧, 傅滨, 肖玉梅, 覃兆海*( )
)
收稿日期:2025-07-26
修回日期:2025-08-22
发布日期:2025-09-24
作者简介:† 共同第一作者
基金资助:
Sai Hong, Fahong Yin, Minghui Chen, Bin Fu, Yumei Xiao, Zhaohai Qin*( )
)
Received:2025-07-26
Revised:2025-08-22
Published:2025-09-24
Contact:
* E-mail: qinzhaohai@263.net
About author:† The authors contributed equally to this work
Supported by:Share
Sai Hong, Fahong Yin, Minghui Chen, Bin Fu, Yumei Xiao, Zhaohai Qin. Study on the Synthesis and Fungicidal Activity of 1-Azolyl-1,1- diarylmethanes Containing Pyridine Ring[J]. Chinese Journal of Organic Chemistry, 2026, 46(1): 106-117.
| Compd. | Inhibition rate/(%, 50 mg/L) | MIRc % | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Log Pa | R. solanib | S. sclerotiorumb | P. capsicib | C. gloeosporioidesb | F. oxysporumb | F. graminearumb | F. fujikuroib | P. aphanidermatumb | B. cinereab | P. oryzaeb | ||
| I-1 | 3.42 | 22.15±2.51 | 16.80±2.08 | 8.74±0.93 | 18.68±0.54 | 12.18±0.84 | 45.05±0.79 | 25.77±2.43 | 17.37±1.24 | 28.14±1.89 | 50.10±2.15 | 24.5 |
| I-2 | 3.84 | 35.64±2.64 | 29.23±1.98 | 26.81±0.73 | 33.50±0.36 | 26.98±0.42 | 53.91±1.42 | 37.99±3.02 | 31.75±0.35 | 45.08±1.92 | 44.66±1.31 | 36.56 |
| I-3 | 4.25 | 57.46±1.06 | 41.00±0.60 | 55.71±0.20 | 43.34±0.74 | 36.92±0.56 | 57.43±0.34 | 38.22±2.22 | 33.88±1.22 | 63.22±2.16 | 49.28±2.33 | 47.65 |
| I-4 | 4.17 | 60.46±0.43 | 32.10±1.71 | 44.76±0.35 | 43.69±0.41 | 39.11±2.22 | 57.31±1.42 | 51.48±1.40 | 39.77±0.54 | 45.65±0.52 | 36.10±0.57 | 45.04 |
| I-5 | 5.09 | 57.57±1.19 | 43.62±2.67 | 60.49±0.93 | 44.64±1.03 | 41.41±0.63 | 61.85±1.23 | 48.02±0.87 | 42.48±0.41 | 84.41±2.03 | 57.84±3.18 | 54.23 |
| I-6 | 4.64 | 63.82±0.87 | 48.85±0.45 | 57.23±0.88 | 44.40±2.05 | 47.72±0.21 | 62.76±1.04 | 55.74±0.69 | 46.72±0.41 | 58.42±1.04 | 21.61±0.29 | 50.73 |
| I-7 | 5.05 | 65.79±2.81 | 50.16±0.78 | 57.69±0.35 | 48.91±0.21 | 45.05±1.31 | 76.61±2.39 | 55.86±0.87 | 49.08±1.41 | 68.36±1.04 | 26.22±3.21 | 54.37 |
| I-8 | 3.49 | 18.75±3.79 | 17.19±1.18 | 21.45±0.81 | 25.56±0.54 | 20.18±0.42 | 39.03±1.48 | 37.76±3.99 | 33.40±2.84 | 47.12±2.06 | 75.46±0.29 | 33.59 |
| I-9 | 3.09 | 3.18±2.74 | 0.31±0.78 | 2.80±0.70 | 12.40±0.54 | 8.90±0.56 | 37.78±0.39 | 33.15±1.60 | 33.52±1.22 | 44.18±2.07 | 34.29±0.49 | 21.05 |
| I-10 | 3.76 | 21.82±2.19 | 23.99±1.77 | 31.24±0.88 | 32.08±0.71 | 23.70±1.52 | 42.55±0.39 | 45.37±1.20 | 35.29±1.06 | 39.44±1.41 | 78.26±0.99 | 37.37 |
| I-11 | 4.92 | 60.20±0.87 | 54.87±2.08 | 59.91±1.41 | 50.57±0.62 | 37.41±0.36 | 55.95±0.79 | 46.52±1.06 | 53.32±0.61 | 86.78±3.11 | 41.04±0.57 | 54.66 |
| I-12 | 4.61 | 64.80±1.51 | 67.03±0.39 | 53.15±1.95 | 53.77±0.36 | 43.84±0.21 | 75.25±0.52 | 51.48±0.20 | 77.84±0.82 | 88.25±0.85 | 82.54±1.03 | 65.8 |
| I-13 | 2.80 | 15.90±1.55 | 7.12±0.23 | 0.00 | 12.87±0.36 | 8.18±0.21 | 31.77±0.20 | 21.39±2.22 | 15.37±0.41 | 58.87±3.20 | 29.02±1.24 | 20.05 |
| I-14 | 3.91 | 26.75±4.06 | 25.95±0.23 | 21.45±0.53 | 31.96±0.21 | 21.88±1.83 | 56.18±0.39 | 30.61±3.73 | 21.97±0.74 | 18.42±0.39 | 51.91±0.57 | 30.71 |
| I-15 | 3.91 | 19.19±2.33 | 17.32±2.61 | 18.88±1.05 | 21.76±0.62 | 19.46±0.42 | 31.77±1.29 | 25.54±3.81 | 15.49±0.61 | 55.14±1.09 | 38.24±1.71 | 26.28 |
| I-16 | 3.91 | 3.29±2.37 | 8.69±0.82 | 16.90±1.58 | 20.46±0.21 | 15.21±7.31 | 35.51±1.42 | 16.55±2.69 | 12.78±1.14 | 38.64±1.55 | 46.64±0.99 | 21.47 |
| I-17 | 4.29 | 52.96±3.47 | 44.40±0.82 | 50.70±1.26 | 39.90±0.62 | 36.44±1.11 | 48.23±1.77 | 48.48±2.42 | 27.39±0.74 | 52.09±2.18 | 28.19±3.02 | 42.88 |
| I-18 | 4.64 | 54.82±1.52 | 49.63±2.16 | 53.73±0.73 | 44.64±1.03 | 35.10±0.42 | 49.25±0.59 | 42.95±1.38 | 46.49±1.08 | 66.10±1.89 | 27.54±1.51 | 47.03 |
| I-19 | 5.34 | 68.75±0.33 | 65.72±1.59 | 59.91±1.07 | 40.61±1.23 | 61.67±0.76 | 68.44±1.94 | 59.77±0.53 | 48.14±1.47 | 73.79±2.74 | 24.74±2.00 | 57.15 |
| I-20 | 4.64 | 41.34±1.55 | 24.65±0.78 | 39.42±1.15 | 28.99±0.21 | 25.16±1.38 | 48.00±1.71 | 31.77±2.22 | 29.40±0.20 | 47.29±0.24 | 23.09±2.00 | 33.91 |
| I-21 | 5.63 | 65.24±1.33 | 72.53±1.04 | 45.34±1.41 | 46.42±0.21 | 31.59±1.67 | 70.59±0.20 | 42.25±0.35 | 32.11±2.55 | 88.93±0.39 | 43.02±0.57 | 53.8 |
| I-22 | 3.07 | 48.03±3.01 | 0.00 | 24.94±1.01 | 33.14±1.23 | 12.54±2.00 | 56.18±0.39 | 34.65±1.04 | 18.79±2.13 | 0.00 | 74.80±1.31 | 30.31 |
| I-23 | 4.28 | 66.01±1.00 | 11.43±0.45 | 87.18±0.73 | 52.11±0.54 | 28.68±1.89 | 79.11±2.58 | 52.51±1.00 | 84.21±0.82 | 49.04±3.43 | 53.15±2.45 | 56.34 |
| I-24 | 3.55 | 40.24±2.64 | 1.88±0.78 | 34.73±2.27 | 40.02±0.21 | 16.06±0.76 | 61.06±0.52 | 55.62±0.53 | 8.06±0.71 | 6.67±0.85 | 71.34±1.48 | 33.57 |
| Bifonazole | 4.69 | 75.44±4.49 | 90.71±1.59 | 96.39±1.58 | 94.90±0.21 | 91.51±1.17 | 92.28±0.86 | 80.06±0.20 | 72.87±0.38 | 100.00 | 100.00 | 89.42 |
| Compd. | Inhibition rate/(%, 50 mg/L) | MIRc % | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Log Pa | R. solanib | S. sclerotiorumb | P. capsicib | C. gloeosporioidesb | F. oxysporumb | F. graminearumb | F. fujikuroib | P. aphanidermatumb | B. cinereab | P. oryzaeb | ||
| I-1 | 3.42 | 22.15±2.51 | 16.80±2.08 | 8.74±0.93 | 18.68±0.54 | 12.18±0.84 | 45.05±0.79 | 25.77±2.43 | 17.37±1.24 | 28.14±1.89 | 50.10±2.15 | 24.5 |
| I-2 | 3.84 | 35.64±2.64 | 29.23±1.98 | 26.81±0.73 | 33.50±0.36 | 26.98±0.42 | 53.91±1.42 | 37.99±3.02 | 31.75±0.35 | 45.08±1.92 | 44.66±1.31 | 36.56 |
| I-3 | 4.25 | 57.46±1.06 | 41.00±0.60 | 55.71±0.20 | 43.34±0.74 | 36.92±0.56 | 57.43±0.34 | 38.22±2.22 | 33.88±1.22 | 63.22±2.16 | 49.28±2.33 | 47.65 |
| I-4 | 4.17 | 60.46±0.43 | 32.10±1.71 | 44.76±0.35 | 43.69±0.41 | 39.11±2.22 | 57.31±1.42 | 51.48±1.40 | 39.77±0.54 | 45.65±0.52 | 36.10±0.57 | 45.04 |
| I-5 | 5.09 | 57.57±1.19 | 43.62±2.67 | 60.49±0.93 | 44.64±1.03 | 41.41±0.63 | 61.85±1.23 | 48.02±0.87 | 42.48±0.41 | 84.41±2.03 | 57.84±3.18 | 54.23 |
| I-6 | 4.64 | 63.82±0.87 | 48.85±0.45 | 57.23±0.88 | 44.40±2.05 | 47.72±0.21 | 62.76±1.04 | 55.74±0.69 | 46.72±0.41 | 58.42±1.04 | 21.61±0.29 | 50.73 |
| I-7 | 5.05 | 65.79±2.81 | 50.16±0.78 | 57.69±0.35 | 48.91±0.21 | 45.05±1.31 | 76.61±2.39 | 55.86±0.87 | 49.08±1.41 | 68.36±1.04 | 26.22±3.21 | 54.37 |
| I-8 | 3.49 | 18.75±3.79 | 17.19±1.18 | 21.45±0.81 | 25.56±0.54 | 20.18±0.42 | 39.03±1.48 | 37.76±3.99 | 33.40±2.84 | 47.12±2.06 | 75.46±0.29 | 33.59 |
| I-9 | 3.09 | 3.18±2.74 | 0.31±0.78 | 2.80±0.70 | 12.40±0.54 | 8.90±0.56 | 37.78±0.39 | 33.15±1.60 | 33.52±1.22 | 44.18±2.07 | 34.29±0.49 | 21.05 |
| I-10 | 3.76 | 21.82±2.19 | 23.99±1.77 | 31.24±0.88 | 32.08±0.71 | 23.70±1.52 | 42.55±0.39 | 45.37±1.20 | 35.29±1.06 | 39.44±1.41 | 78.26±0.99 | 37.37 |
| I-11 | 4.92 | 60.20±0.87 | 54.87±2.08 | 59.91±1.41 | 50.57±0.62 | 37.41±0.36 | 55.95±0.79 | 46.52±1.06 | 53.32±0.61 | 86.78±3.11 | 41.04±0.57 | 54.66 |
| I-12 | 4.61 | 64.80±1.51 | 67.03±0.39 | 53.15±1.95 | 53.77±0.36 | 43.84±0.21 | 75.25±0.52 | 51.48±0.20 | 77.84±0.82 | 88.25±0.85 | 82.54±1.03 | 65.8 |
| I-13 | 2.80 | 15.90±1.55 | 7.12±0.23 | 0.00 | 12.87±0.36 | 8.18±0.21 | 31.77±0.20 | 21.39±2.22 | 15.37±0.41 | 58.87±3.20 | 29.02±1.24 | 20.05 |
| I-14 | 3.91 | 26.75±4.06 | 25.95±0.23 | 21.45±0.53 | 31.96±0.21 | 21.88±1.83 | 56.18±0.39 | 30.61±3.73 | 21.97±0.74 | 18.42±0.39 | 51.91±0.57 | 30.71 |
| I-15 | 3.91 | 19.19±2.33 | 17.32±2.61 | 18.88±1.05 | 21.76±0.62 | 19.46±0.42 | 31.77±1.29 | 25.54±3.81 | 15.49±0.61 | 55.14±1.09 | 38.24±1.71 | 26.28 |
| I-16 | 3.91 | 3.29±2.37 | 8.69±0.82 | 16.90±1.58 | 20.46±0.21 | 15.21±7.31 | 35.51±1.42 | 16.55±2.69 | 12.78±1.14 | 38.64±1.55 | 46.64±0.99 | 21.47 |
| I-17 | 4.29 | 52.96±3.47 | 44.40±0.82 | 50.70±1.26 | 39.90±0.62 | 36.44±1.11 | 48.23±1.77 | 48.48±2.42 | 27.39±0.74 | 52.09±2.18 | 28.19±3.02 | 42.88 |
| I-18 | 4.64 | 54.82±1.52 | 49.63±2.16 | 53.73±0.73 | 44.64±1.03 | 35.10±0.42 | 49.25±0.59 | 42.95±1.38 | 46.49±1.08 | 66.10±1.89 | 27.54±1.51 | 47.03 |
| I-19 | 5.34 | 68.75±0.33 | 65.72±1.59 | 59.91±1.07 | 40.61±1.23 | 61.67±0.76 | 68.44±1.94 | 59.77±0.53 | 48.14±1.47 | 73.79±2.74 | 24.74±2.00 | 57.15 |
| I-20 | 4.64 | 41.34±1.55 | 24.65±0.78 | 39.42±1.15 | 28.99±0.21 | 25.16±1.38 | 48.00±1.71 | 31.77±2.22 | 29.40±0.20 | 47.29±0.24 | 23.09±2.00 | 33.91 |
| I-21 | 5.63 | 65.24±1.33 | 72.53±1.04 | 45.34±1.41 | 46.42±0.21 | 31.59±1.67 | 70.59±0.20 | 42.25±0.35 | 32.11±2.55 | 88.93±0.39 | 43.02±0.57 | 53.8 |
| I-22 | 3.07 | 48.03±3.01 | 0.00 | 24.94±1.01 | 33.14±1.23 | 12.54±2.00 | 56.18±0.39 | 34.65±1.04 | 18.79±2.13 | 0.00 | 74.80±1.31 | 30.31 |
| I-23 | 4.28 | 66.01±1.00 | 11.43±0.45 | 87.18±0.73 | 52.11±0.54 | 28.68±1.89 | 79.11±2.58 | 52.51±1.00 | 84.21±0.82 | 49.04±3.43 | 53.15±2.45 | 56.34 |
| I-24 | 3.55 | 40.24±2.64 | 1.88±0.78 | 34.73±2.27 | 40.02±0.21 | 16.06±0.76 | 61.06±0.52 | 55.62±0.53 | 8.06±0.71 | 6.67±0.85 | 71.34±1.48 | 33.57 |
| Bifonazole | 4.69 | 75.44±4.49 | 90.71±1.59 | 96.39±1.58 | 94.90±0.21 | 91.51±1.17 | 92.28±0.86 | 80.06±0.20 | 72.87±0.38 | 100.00 | 100.00 | 89.42 |
| Compd. | EC50 | Regression equation | R2 | 95% confidence interval | |
|---|---|---|---|---|---|
| P. oryzae | I-08 | 49.17 | y=-2.54+1.48x | 0.941 | 38.18~70.10 |
| I-10 | 33.34 | y=-2.21+1.44x | 0.984 | 26.81~43.90 | |
| I-12 | 15.67 | y=-1.91+1.59x | 0.985 | 13.20~18.93 | |
| I-22 | 46.03 | y=-2.4+1.45x | 0.960 | 35.31~65.86 | |
| I-24 | 20.77 | y=-1.75+1.33x | 0.997 | 16.87~26.60 | |
| Bifonazole | 1.41 | y=-0.21+1.49x | 0.947 | 0.72~2.12 | |
| B. cinerea | I-05 | 15.25 | y=-1.97+1.66x | 0.992 | 12.94~18.24 |
| I-11 | 14.21 | y=-1.4+1.21x | 0.985 | 11.41~17.92 | |
| I-12 | 21.65 | y=-2.09+1.56x | 0.970 | 18.15~26.57 | |
| I-19 | 22.01 | y=-1.82+1.35x | 0.992 | 17.92~28.13 | |
| I-21 | 16.41 | y=-1.85+1.51x | 0.982 | 13.72~20.02 | |
| Bifonazole | 1.78 | y=-0.38+1.41x | 0.997 | 0.75~3.00 | |
| F. graminearum | I-07 | 22.84 | y=-1.21+0.89x | 0.988 | 16.88~34.18 |
| I-12 | 17.3 | y=-1.15+0.93x | 0.996 | 13.20~24.12 | |
| I-21 | 19.71 | y=-0.76+0.59x | 0.996 | 12.97~36.65 | |
| I-23 | 12.76 | y=-0.78+0.71x | 0.991 | 9.12~18.98 | |
| Bifonazole | 0.1 | y=0.76+0.77x | 0.920 | 0.08~0.15 | |
| P. aphanidermatum | I-12 | 27.54 | y=-2.68+1.86x | 0.976 | 23.41~33.34 |
| I-23 | 14.03 | y=-2.73+2.35x | 0.963 | 9.87~20.48 | |
| Bifonazole | 14.87 | y=-1.97+1.66x | 0.996 | 10.94~21.28 | |
| P. capsici | I-23 | 20.17 | y=-3.95+3.02x | 0.997 | 18.07~22.62 |
| Bifonazole | 6.5 | y=-2.59+3.11x | 0.985 | 5.83~7.23 | |
| S. sclerotiorum | I-21 | 16.53 | y=-1.93+1.57x | 0.963 | 14.00~19.86 |
| Bifonazole | 11.94 | y=-2.01+1.89x | 0.984 | 10.36~13.84 |
| Compd. | EC50 | Regression equation | R2 | 95% confidence interval | |
|---|---|---|---|---|---|
| P. oryzae | I-08 | 49.17 | y=-2.54+1.48x | 0.941 | 38.18~70.10 |
| I-10 | 33.34 | y=-2.21+1.44x | 0.984 | 26.81~43.90 | |
| I-12 | 15.67 | y=-1.91+1.59x | 0.985 | 13.20~18.93 | |
| I-22 | 46.03 | y=-2.4+1.45x | 0.960 | 35.31~65.86 | |
| I-24 | 20.77 | y=-1.75+1.33x | 0.997 | 16.87~26.60 | |
| Bifonazole | 1.41 | y=-0.21+1.49x | 0.947 | 0.72~2.12 | |
| B. cinerea | I-05 | 15.25 | y=-1.97+1.66x | 0.992 | 12.94~18.24 |
| I-11 | 14.21 | y=-1.4+1.21x | 0.985 | 11.41~17.92 | |
| I-12 | 21.65 | y=-2.09+1.56x | 0.970 | 18.15~26.57 | |
| I-19 | 22.01 | y=-1.82+1.35x | 0.992 | 17.92~28.13 | |
| I-21 | 16.41 | y=-1.85+1.51x | 0.982 | 13.72~20.02 | |
| Bifonazole | 1.78 | y=-0.38+1.41x | 0.997 | 0.75~3.00 | |
| F. graminearum | I-07 | 22.84 | y=-1.21+0.89x | 0.988 | 16.88~34.18 |
| I-12 | 17.3 | y=-1.15+0.93x | 0.996 | 13.20~24.12 | |
| I-21 | 19.71 | y=-0.76+0.59x | 0.996 | 12.97~36.65 | |
| I-23 | 12.76 | y=-0.78+0.71x | 0.991 | 9.12~18.98 | |
| Bifonazole | 0.1 | y=0.76+0.77x | 0.920 | 0.08~0.15 | |
| P. aphanidermatum | I-12 | 27.54 | y=-2.68+1.86x | 0.976 | 23.41~33.34 |
| I-23 | 14.03 | y=-2.73+2.35x | 0.963 | 9.87~20.48 | |
| Bifonazole | 14.87 | y=-1.97+1.66x | 0.996 | 10.94~21.28 | |
| P. capsici | I-23 | 20.17 | y=-3.95+3.02x | 0.997 | 18.07~22.62 |
| Bifonazole | 6.5 | y=-2.59+3.11x | 0.985 | 5.83~7.23 | |
| S. sclerotiorum | I-21 | 16.53 | y=-1.93+1.57x | 0.963 | 14.00~19.86 |
| Bifonazole | 11.94 | y=-2.01+1.89x | 0.984 | 10.36~13.84 |
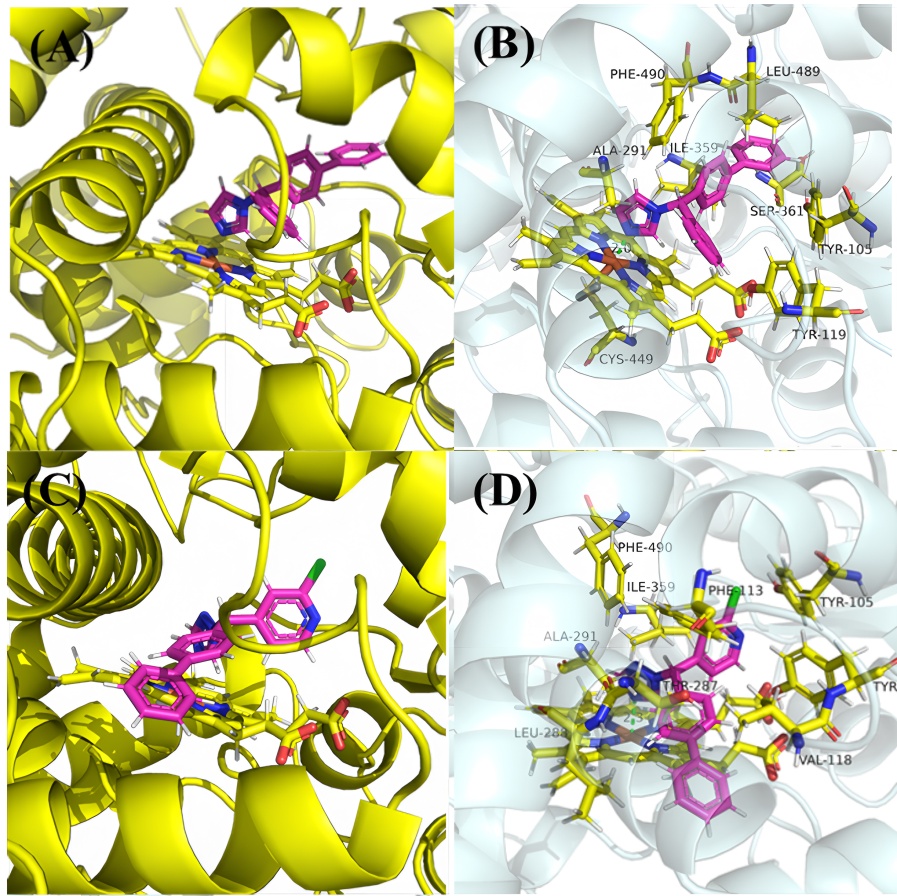
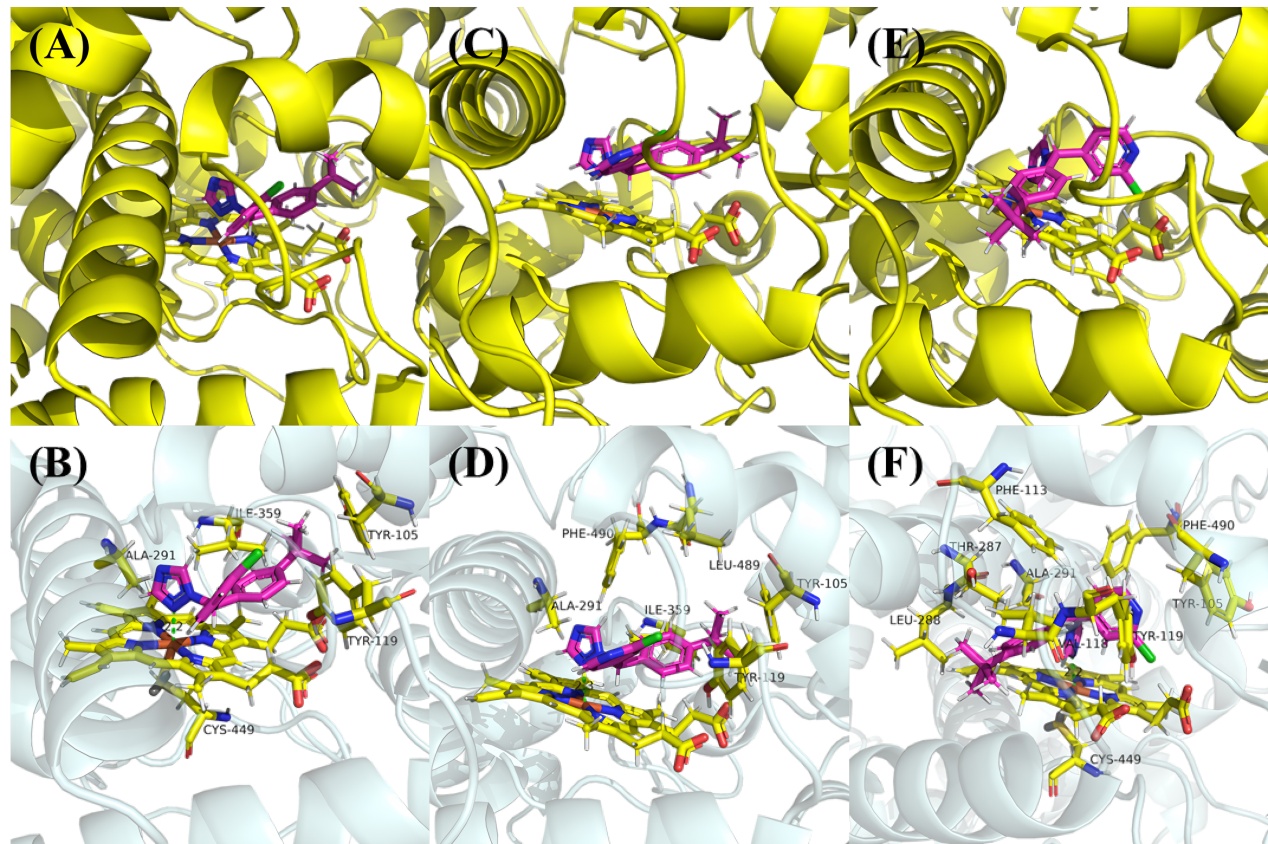
| [1] |
doi: 10.1002/jhet.v61.2 |
| [2] |
doi: 10.1039/C9CS00556K |
| [3] |
doi: 10.2217/fmb-2021-0173 pmid: 34783586 |
| [4] |
doi: 10.1016/j.envpol.2022.119553 |
| [5] |
doi: 10.1016/j.envpol.2019.03.067 |
| [6] |
pmid: 16466539 |
| [7] |
doi: 10.1007/s12154-008-0010-6 pmid: 19568799 |
| [8] |
doi: 10.1016/j.bbapap.2010.06.006 pmid: 20547249 |
| [9] |
doi: 10.1016/0048-3575(73)90005-9 |
| [10] |
doi: 10.1074/jbc.M110.133215 pmid: 20530488 |
| [11] |
doi: 10.1016/j.bbagen.2006.07.018 pmid: 16963187 |
| [12] |
|
| [13] |
doi: 10.1016/j.ejmech.2023.115658 |
| [14] |
doi: 10.1002/ps.v26:2 |
| [15] |
doi: 10.1016/j.aac.2024.10.002 |
| [16] |
|
| [17] |
doi: 10.1016/j.postharvbio.2010.08.017 |
| [18] |
doi: 10.1016/j.pestbp.2018.11.013 |
| [19] |
doi: 10.1016/j.fgb.2016.04.003 |
| [20] |
doi: 10.1021/acs.jafc.3c09543 |
| [21] |
doi: 10.1016/j.pestbp.2022.105169 |
| [22] |
doi: 10.1002/ps.v81.4 |
| [23] |
doi: 10.1021/acs.jafc.4c10490 |
| [24] |
doi: 10.1021/acs.jmedchem.4c00032 |
| [25] |
doi: 10.3390/jof10020160 |
| [26] |
pmid: 2670516 |
| [27] |
pmid: 2528693 |
| [28] |
|
| [29] |
|
|
(车传亮, 杨冬燕, 万川, 王家尧, 刘雪莲, 赵峰海, 覃兆海, 农药学学报, 2017, 19, 533.)
|
|
| [30] |
|
|
(慕长炜, 袁会珠, 李楠, 傅滨, 肖玉梅, 马永强, 齐淑华, 覃兆海, 高等学校化学学报, 2007, 1902.)
|
|
| [31] |
doi: 10.1021/jf902410x |
| [32] |
doi: 10.3390/molecules16118945 |
| [33] |
doi: 10.1002/ps.v76.6 |
| [34] |
doi: 10.1016/j.tet.2018.04.066 |
| [35] |
doi: 10.1021/ac501780z pmid: 25098642 |
| [36] |
doi: 10.1021/jacs.1c00529 pmid: 33655746 |
| [37] |
|
|
(孙家隆, 慕卫, 农药实验技术与指导, 化学工业出版社, 北京, 2009.)
|
|
| [38] |
doi: 10.1002/jcc.21334 pmid: 19499576 |
| [1] | Xinyi Cui, Lifan Guo, Congxuan Ma, Yun Li, Jianhua Liang. Structural Modifications, Structure-Activity Relationships, and Total Synthesis Advances in Erythromycin Analogs against Resistant Pathogens [J]. Chinese Journal of Organic Chemistry, 2026, 46(1): 39-73. |
| [2] | Rui Zhang, Yin Zhang, Wenjuan Li, Xiaoqiang Han, Jixing Zhao. Construction and Antifungal Activity Study of Trifluoroacetyl Substituted Spiro Indolinone-Hexahydropyrrolizine Derivatives [J]. Chinese Journal of Organic Chemistry, 2025, 45(9): 3378-3391. |
| [3] | Youxue Zhao, Xiruo Li, Luobing Meng, Chunxiu Li, Guisheng Fan, Jianhe Xu. Advances in Understanding the Substrate Promiscuity of Alcohol Dehydrogenases/Carbonyl Reductases★ [J]. Chinese Journal of Organic Chemistry, 2025, 45(9): 3175-3185. |
| [4] | Haozhe Guo, Yuyin Li, Peichen Tang, Jiangli Fan. Advances in Machine Learning-Based Design of Organic Fluorescent Theranostic Molecules★ [J]. Chinese Journal of Organic Chemistry, 2025, 45(9): 3203-3212. |
| [5] | Min Tang, Bin Zhang, Qiushi Wang, Chaohua Fang, Liwei Hu, Liping Guan. Design, Synthesis and Biological Activity Study of Chalcone Derivatives Based on the Inhibitory Activities of Monoamine Oxidase and Cholinesterase Containing Indole Ring and Benzothiophene Ring [J]. Chinese Journal of Organic Chemistry, 2025, 45(8): 2989-3003. |
| [6] | Juanjuan Liu, Ya Gao, Guoyong Luo, Shaoping Yang. Design, Synthesis, Antitumor Activities and Molecular Docking of N-Isopimaroyl-N'-aroyl Thiosemicarbazide Derivatives [J]. Chinese Journal of Organic Chemistry, 2025, 45(12): 4497-4504. |
| [7] | Dongping Qiu, Zhaoxu Wang, Jie Zhang, Liang Guo. Design, Synthesis and Antitumor Activity of Novel Imide-β-carboline [J]. Chinese Journal of Organic Chemistry, 2025, 45(12): 4362-4374. |
| [8] | Yitao Yan, Yinglu Chen, Hanxian Hu, Jun Wu. Synthesis and Herbicidal Activity of Di-substituted Pyrimidine-Biphenyls and Study of Molecular Mode of Action [J]. Chinese Journal of Organic Chemistry, 2025, 45(1): 358-366. |
| [9] | Haiping Tian, Dongdong Liu, Hongyan Pei, Jialin Ye, Zirui Zheng, Yixing Gao, Changxing Li, Huan Tian, Jing Zhang, Lixin Zhang. Design, Synthesis and Insecticidal Activity of New Phenylpyrazole Derivatives [J]. Chinese Journal of Organic Chemistry, 2025, 45(1): 227-239. |
| [10] | Baifeng Zheng, Yang Zuo, Qiong Chen, Qiongyou Wu. Design, Synthesis and Herbicidal Activity of Novel Benzothiazole-Pyrimidinediones Compounds [J]. Chinese Journal of Organic Chemistry, 2024, 44(7): 2371-2376. |
| [11] | Liqing Qin, Guishan Lin, Wengui Duan, Yucheng Cui, Maofang Yang, Fangyao Li, Dianpeng Li. Synthesis, Antiproliferative Activity, 3D-QSAR and Molecular Docking Study of Novel Longifolene-Derived Tetraline Fused N-Acyl-pyrazole Compounds [J]. Chinese Journal of Organic Chemistry, 2024, 44(6): 1967-1977. |
| [12] | Guochao Liang, Tingting Dong, Haiying Ji, Chunyan Wang, Yali Song, Wei Zhang. Synthesis and Antitumor Activity of Novel 3,3'-((4-Chloro-2H-thiochromen-3-yl)methylene)bis(1H-indole)-Like Topoisomerase II Inhibitors [J]. Chinese Journal of Organic Chemistry, 2024, 44(6): 1949-1956. |
| [13] | Yiming Hu, Jiayu Xu, Min Tang, Yawen Liu, Liping Guan, Qinghao Jin. Design, Synthesis and Biological Activity Studies of 2-(1,3-Dioxoiso-indolin-2-yl)-N-phenethylacetamide and 2-(3,4-Dihydroisoquinolin-1-yl)isoindole-1,3-dione as Monoamine Oxidase (MAO) and Cholinesterase (ChE) Inhibitors [J]. Chinese Journal of Organic Chemistry, 2024, 44(6): 1907-1919. |
| [14] | Jiyong Liu, Minghui Wu, Juncheng Xiang, Huailin Pang, Bin Li, Liang Lü. Synthesis, Insecticidal Activities, and Structure-Activity Relationship Studies of Novel Diamide Compounds Containing (Halogenated) Alkoxy Group [J]. Chinese Journal of Organic Chemistry, 2024, 44(5): 1584-1591. |
| [15] | Hongyan Pei, Jialin Ye, Feng Wang, Dongdong Liu, Yukui Yu, Jing Zhang, Lixin Zhang. Design, Synthesis and Herbicidal Activity of Novel Uracil Compounds Containing Piperidine Moiety [J]. Chinese Journal of Organic Chemistry, 2024, 44(5): 1592-1605. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||