Chinese Journal of Organic Chemistry ›› 2021, Vol. 41 ›› Issue (1): 12-51.DOI: 10.6023/cjoc202007045 Previous Articles Next Articles
Special Issue: 热点论文虚拟合集
REVIEWS
王乐乐a, 张子莹a, 韩华彬a, 刘雄利b, 卜站伟a, 王琪琳a,*( )
)
收稿日期:2020-07-18
修回日期:2020-08-29
发布日期:2020-09-16
通讯作者:
王琪琳
作者简介:基金资助:
Lele Wanga, Ziying Zhanga, Huabin Hana, Xiongli Liub, Zhanwei Bua, Qilin Wanga,*( )
)
Received:2020-07-18
Revised:2020-08-29
Published:2020-09-16
Contact:
Qilin Wang
Supported by:Share
Lele Wang, Ziying Zhang, Huabin Han, Xiongli Liu, Zhanwei Bu, Qilin Wang. Recent Advances in the Construction of Bridged Rings through Cycloadditions and Cascade Reactions[J]. Chinese Journal of Organic Chemistry, 2021, 41(1): 12-51.
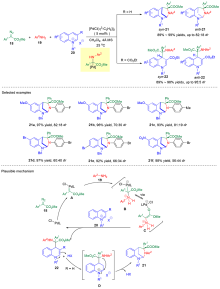
| [1] |
(a) Thopate S.B.; Jadhav S.B.; Nanubolu J.B.; Chegondi R. ACS Catal. 2019, 9, 10012.
|
|
(b) Gouse S.; Reddy N.R.; Baskaran S. Org. Lett. 2019, 21, 3822.
|
|
|
(c) Liu Z.G.; Meng Y.F.; Yuan P.R.; Wang Z.S.; Gao J.M.; Zheng H.J. Org. Lett. 2020, 22, 520.
|
|
| [2] |
Chao C.H.; Cheng J.C.; Shen D.Y.; Wu T.S. J. Nat. Prod. 2014, 77, 22.
|
| [3] |
Xu J.; Wang M.C.; Sun X.S.; Ren Q.H.; Cao X.R.; Li S.; Su G.C.; Tuerhong M.; Lee D.H.; Ohizumi Y.; Bartlam M.; Guo Y.Q. J. Nat. Prod. 2016, 79, 2924.
|
| [4] |
(a) Yan Y.M.; Ai J.; Zhou L.L.; Chung A.C.K.; Li R.; Nie J.; Fang P.; Wang X.L.; Luo J.; Hu Q.; Hou F.F.; Cheng Y.X. Org. Lett. 2013, 15, 5488.
|
|
(b) Riehl P.S.; Richardson A.D.; Sakamoto T.; Schindler C.S. Org. Lett. 2020, 22, 290.
|
|
| [5] |
(a) Carroll A.R.; Hyde E.; Smith J.; Quinn R.J.; Guymer G.; Forster P.I. J. Org. Chem. 2005, 70, 1096.
|
|
(b) Martin C.L.; Overman L.E.; Rohde J.M. J. Am. Chem. Soc. 2008, 130, 7568.
|
|
| [6] |
Bera S.; Chatterjee B.; Mondal D. Eur. J. Org. Chem. 2018, 5337.
|
| [7] |
(a) Shan D.; Jia Y.X. Chin. J. Org. Chem. 2013, 33, 1144.
|
|
( 单东, 贾彦兴, 有机化学, 2013, 33, 1144.).
|
|
|
(b) Yuan K.; Jia Y.X. Chin. J. Org. Chem. 2018, 38, 2386.
|
|
|
( 袁括, 贾彦兴, 有机化学, 2018, 38, 2386.).
|
|
| [8] |
(a) Liu J.Y.; Liu X.; Wu J.L.; Li C.C. Chem 2020, 6, 579.
|
|
(b) Min L.; Liu X.; Li C.C. Acc. Chem. Res. 2020, 53, 703.
|
|
|
(c) Liu X.; Hu Y.J.; Fan J.H.; Zhao J.; Li S.P.; Li C.C. Org. Chem. Front. 2018, 5, 1217.
|
|
| [9] |
(a) Mu W.H.; Cheng R.J.; Shang Y.W.; He R.Z.; Li D.L.; Fu M. Chin. J. Org. Chem. 2018, 38, 1327.
|
|
( 母伟花, 程瑞姣, 商英伟, 贺仁泽, 李冬丽, 傅冕, 有机化学, 2018, 38, 1327.).
|
|
|
(b) Feng T.; Du J.; Yan W.J.; Li X.; Gao W.C.; Chang H.H.; Wei W.L. Chin. J. Org. Chem. 2019, 39, 1197.
|
|
|
( 冯涛, 杜佳, 闫文静, 李兴, 高文超, 常宏宏, 魏文珑, 有机化学, 2019, 39, 1197.).
|
|
|
(c) Cai Q. Chin. J. Chem. 2019, 37, 946.
|
|
|
(d) Zhang Y.C.; Jiang F.; Shi F. Acc. Chem. Res. 2020, 53, 425.
|
|
| [10] |
(a) O’Hagan D. Nat. Prod. Rep. 2000, 17, 435.
|
|
(b) Cheenpracha S.; Ritthiwigrom T.; Laphookhieo S. J. Nat. Prod. 2013, 76, 723.
|
|
| [11] |
Narayan R.; Bauer J.O.; Strohmann C.; Antonchick A.P.; Waldmann H. Angew. Chem., Int. Ed. 2013, 52, 12892.
|
| [12] |
Wang Z.; Wang D.C.; Xie M.S.; Qu G.R.; Guo H.M. Org. Lett. 2020, 22, 164.
|
| [13] |
Jia Z.J.; Shan G.; Daniliuc C.G.; Antonchick A.P.; Waldmann H. Angew. Chem., Int. Ed. 2018, 57, 14493.
|
| [14] |
Suga H.; Yoshiwara M.; Yamaguchi T.; Bando T.; Taguchi M.; Inaba A.; Goto Y.; Kikuchi A.; Itoh K.; Toda Y. Chem. Commun. 2019, 55, 1552.
|
| [15] |
Kang Z.H.; Zhang D.; Hu W.H. Org. Lett. 2017, 19, 3783.
|
| [16] |
Leitch J.A.; Rogova T.; Duarte F.; Dixon D.J. Angew. Chem., Int. Ed. 2020, 59, 4121.
|
| [17] |
Liu X.Y.; Yin Y.L.; Jiang Z.Y. Chem. Commun. 2019, 55, 11527.
|
| [18] |
(a) Rao Y.; Yin G.D. Org. Biomol. Chem. 2013, 11, 6029.
|
|
(b) Gao Y.Q.; Hou Y.; Zhu L.M.; Chen G.Z.; Xu D.Y.; Zhang S.Y.; He Y.P.; Xie W.Q. RSC Adv. 2019, 9, 29005.
|
|
| [19] |
Zhu Y.S.; Zhou J.; Jin S.J.; Dong H.H.; Guo J.M.; Bai X.G.; Wang Q.L.; Bu Z.W. Chem. Commun. 2017, 53, 11201.
|
| [20] |
Zhu Y.S.; Guo J.; Jin S.J.; Guo J.M.; Bai X.G.; Wang Q.L.; Bu Z.W. Org. Biomol. Chem. 2018, 16, 1751.
|
| [21] |
Jin S.J.; Guo J.; Fang D.M.; Huang Y.W.; Wang Q.L.; Bu Z.W. Adv. Synth. Catal. 2019, 361, 456.
|
| [22] |
Balha M.; Pan S.C. J. Org. Chem. 2018, 83, 14703.
|
| [23] |
Balha M.; Soni C.; Pan S.C. Eur. J. Org. Chem. 2019, 2552.
|
| [24] |
Ramachary D.B.; Anif Pasha M.; Thirupathi G. Angew. Chem., Int. Ed. 2017, 56, 12930.
|
| [25] |
Cui R.R.; Ye J.X.; Li J.; Mo W.H.; Gao Y.; Chen H.J. Org. Lett. 2020, 2 2, 116.
|
| [26] |
Buev E.M.; Stepanov M.A.; Moshkin V.S.; Sosnovskikh V.Y. Org. Lett. 2020, 22, 631.
|
| [27] |
Fan W.T.; Li N.K.; Xu L.M.; Qiao C.H.; Wang X.W. Org. Lett. 2017, 19, 6626.
|
| [28] |
Guo J.M.; Bai X.G.; Wang Q.L.; Bu Z.W. J. Org. Chem. 2018, 83, 3679.
|
| [29] |
You Z.H.; Chen Y.H.; Tang Y.; Liu Y.K. Org. Lett. 20 18, 20, 6682.
|
| [30] |
Liu H.X.; Wang Y.; Guo X.Y.; Huo L.Q.; Xu Z.F.; Zhang W.M.; Qiu S.X.; Yang B.; Tan H.B. Org. Lett. 2018, 20, 546.
|
| [31] |
Guo J.M.; Miao H.J.; Zhao Y.; Bai X.G.; Zhu Y.S.; Wang Q.L.; Bu Z.W. Chem. Commun. 2019, 55, 5207.
|
| [32] |
Huo L.Q.; Dong C.M.; Wang M.M.; Lu X.X.; Zhang W.G.; Yang B.; Yuan Y.F.; Qiu S.X.; Liu H.X.; Tan H.B. Org. Lett. 2020, 22, 934.
|
| [33] |
Zheng X.; Yang W.L.; Liu Y.Z.; Wu S.X.; Deng W.P. Adv. Synth. Catal. 2018, 360, 2843.
|
| [34] |
Gao R.D.; Xu Q.L.; Zhang B.; Gu Y.T.; Dao L.X.; You S.L. Chem. -Eur. J. 2016, 22, 11601.
|
| [35] |
Luo M.P.; Chen J.X.; Yu L.Q.; Wei W.G. Eur. J. Org. Chem. 2017, 2017, 2652.
|
| [36] |
Wang W.B.; Bai X.G.; Jin S.J.; Guo J.M.; Zhao Y.; Miao H.J.; Zhu Y.S.; Wang Q.L.; Bu Z.W. Org. Lett. 20 18, 20, 3451.
|
| [37] |
(a) Wang Y.; Li H.M.; Wang Y.Q.; Liu Y.; Foxman B.M.; Deng L. J. Am. Chem. Soc. 2007, 129, 6364.
|
|
(b) Singh R.P.; Bartelson K.; Wang Y.; Su H.; Lu X.J.; Deng L. J. Am. Chem. Soc. 2008, 130, 2422.
|
|
|
(c) Soh J.Y.T.; Tan C.H. J. Am. Chem. Soc. 2009, 131, 6904.
|
|
|
(d) Bartelson K.J.; Singh R.P.; Foxman B.M.; Deng L. Chem. Sci. 2011, 2, 1940.
|
|
| [38] |
Shi L.M.; Dong W.W.; Tao H.Y.; Dong X.Q.; Wang C.J. Org. Lett. 2017, 19, 4532.
|
| [39] |
Cole C.J.F.; Fuentes L.; Snyder S.A. Chem. Sci. 2020, 11, 2175.
|
| [40] |
Saktura M.; Grzelak P.; Dybowska J.; Albrecht, Ɫ.Org. Lett. 2020, 22, 1813.
|
| [41] |
Yarlagadda S.; Sankaram G.S.; Balasubramanian S.; Reddy B.V.S. Org. Lett. 2018, 20, 4195.
|
| [42] |
Katritzky A.R.; Dennis N. Chem. Rev. 1989, 89, 827.
|
| [43] |
Fu C.C.; Lora N.; Kirchhoefer P.L.; Lee D.R.; Altenhofer E.; Barnes C.L.; Hungerford N.L.; Krenske E.H.; Harmata M. Angew. Chem., Int. Ed. 2017, 56, 14682.
|
| [44] |
Villar L.; Uria U.; Martínez J.I.; Prieto L.; Reyes E.; Carrillo L.; Vicario J.L. Angew. Chem., Int. Ed. 2017, 56, 10535.
|
| [45] |
Lam H.; Qureshi Z.; Wegmann M.; Lautens M. Angew. Chem., Int. Ed. 2018, 57, 16185.
|
| [46] |
Xu C.R.; Wang K.X.; Li D.W.; Lin L.L.; Feng X.M. Angew. Chem., Int. Ed. 2019, 58, 18438.
|
| [47] |
Suneja A.; Loui H.J.; Schneider C. Angew. Chem., Int. Ed. 2020, 59, 5536.
|
| [48] |
Reddy K.N.; Chary D.Y.; Sridhar B.; Reddy B.V.S. Org. Lett. 2019, 21, 8548.
|
| [49] |
Feng J.; Zhou M.; Lin X.Z.; Lu A.; Zhang X.Y.; Zhao M. Org. Lett. 2019, 21, 6245.
|
| [50] |
Cheng X.; Cao X.; Zhou S.J.; Cai B.G.; He X.K.; Xuan J. Adv. Synth. Catal. 2019, 361, 1230.
|
| [51] |
Pirovano V.; Brambilla E.; Moretti A.; Rizzato S.; Abbiati G.; Nava D.; Rossi E. J. Org. Chem. 2020, 85, 3265.
|
| [52] |
Wang H.K.; Zeng T.L.; Li X.H.; Wang S.M.; Xiao W.G.; Liu L.Y.; Chang W.X.; Li J. Org. Chem. Front. 2020, 7, 1809.
|
| [53] |
Li Q.; Jiang G.J.; Jiao L.; Yu Z.X. Org. Lett. 2010, 12, 1332.
|
| [54] |
(a) Mei G.J.; Yuan H.; Gu Y.Q.; Chen W.; Chung L.W.; Li C.C. Angew. Chem., Int. Ed. 2014, 53, 11051.
|
|
(b) Mei G.J.; Liu X.; Qiao C.; Chen W.; Li C.C. Angew. Chem., Int. Ed. 2015, 54, 1754.
|
|
| [55] |
Liu J.Y.; Wu J.L.; Fan J.H.; Yan X.; Mei G.J.; Li C.C. J. Am. Chem. Soc. 2018, 140, 5365.
|
| [56] |
Liu X.; Liu J.Y.; Wu J.L.; Huang G.C.; Liang R.; Chung L.W.; Li C.C. J. Am. Chem. Soc. 2019, 141, 2872.
|
| [57] |
Liu X.; Liu J.Y.; Zhao J.; Li S.P.; Li C.C. Org. Lett. 2017, 19, 2742.
|
| [58] |
Xu J.H.; Zheng S.C.; Zhang J.W.; Liu X.Y.; Tan B. Angew. Chem., Int. Ed. 2016, 55, 11834.
|
| [59] |
Hu B.W.; Zhang X.Y.; Mo Y.H.; Li J.Z.; Lin L.L.; Liu X.H.; Feng X.M. Org. Lett. 2020, 22, 1034.
|
| [60] |
Ma Y.L.; Wang K.M.; Huang R.; Lin J.; Yan S.J. Green Chem. 2017, 19, 3574.
|
| [61] |
Guo S.H.; Liu Y.F.; Wang Y.P.; Zhang X.Y.; Fan X.S. J. Org. Chem. 2020, 85, 8910.
|
| [62] |
(a) Zhou J. Chem. -Asian J. 2010, 5, 422.
|
|
(b) Grondal C.; Jeanty M.; Enders D. Nat. Chem. 2010, 2, 167.
|
|
|
(c) Lu L.Q.; Chen J.R.; Xiao W.J. Acc. Chem. Res. 2012, 45, 1278.
|
|
|
(d) Pellissier H. Adv. Synth. Catal. 2012, 354, 237.
|
|
|
(e) Chen D.F.; Han Z.Y.; Zhou X.L.; Gong L.Z. Acc. Chem. Res. 2014, 47, 2365.
|
|
|
(f) Volla C.M.R.; Atodiresei I.; Rueping M. Chem. Rev. 2014, 114, 2390.
|
|
|
(g) Wang Y.; Lu H.; Xu P.F. Acc. Chem. Res. 2015, 48, 1832.
|
|
| [63] |
Tokimizu Y.; Oishi S.; Fujii N.; Ohno H. Angew. Chem. Int. Ed. 2015, 54, 7862.
|
| [64] |
Han Y.P.; Song X.R.; Qiu Y.F.; Li X.S.; Zhang H.R.; Zhu X.Y.; Liu X.Y.; Liang Y.M. Org. Lett. 2016, 18, 3866.
|
| [65] |
Du J.Y.; Ma Y.H.; Meng F.X.; Chen B.L.; Zhang S.L.; Li Q.L.; Gong S.W.; Wang D.Q.; Ma C.L. Org. Lett. 2018, 20, 4371.
|
| [66] |
Ma S.S.; Yu A.M.; Zhang L.; Meng X.T. J. Org. Chem. 2018, 83, 5410.
|
| [67] |
Gurubrahamam R.; Nagaraju K.; Chen K. Chem. Commun. 2018, 54, 6048.
|
| [68] |
Yang S.M.; Karanam P.; Wang M.; Jang Y.J.; Yeh Y.S.; Tseng P.Y.; Ganapuram M.R.; Liou Y.C.; Lin W.W. Chem. Commun. 2019, 55, 1398.
|
| [69] |
Wang C.; Chen Y.H.; Wu H.C.; Wang C.; Liu Y.K. Org. Lett. 2019, 21, 6750.
|
| [70] |
Borade B.R.; Nomula R.; Gonnade R.G.; Kontham R. Org. Lett. 2019, 21, 2629.
|
| [71] |
Jiang B.; Xiao B.X.; Ouyang Q.; Liang H.P.; Du W.; Chen Y.C. Org. Lett. 2019, 21, 3310.
|
| [72] |
Jakkampudi S.; Konda S.; Arman H.; Zhao J.C.G. Adv. Synth. Catal. 2020, 362, 2419.
|
| [73] |
Wang S.D.; Guillot R.; Carpentier J.F.; Sarazin Y.; Bour C.; Gandon V.; Lebœuf D. Angew. Chem., Int. Ed. 2020, 59, 1134.
|
| [74] |
Miao H.J.; Wang L.L.; Han H.B.; Zhao Y.D.; Wang Q.L.; Bu Z.W. Chem. Sci. 2020, 11, 1418.
|
| [75] |
Bai X.G.; Miao, H, J.; Zhao, Y.; Wang, Q.L.; Bu, Z.W.Org. Lett. 202 0, 22, 5068.
|
| [76] |
Wang L.L.; Han H.B.; Cui Z.H.; Zhao J.W.; Bu Z.W.; Wang Q.L. Org. Lett. 2020, 22, 873.
|
| [77] |
Lachkar D.; Denizot N.; Bernadat G.; Ahamada K.; Beniddir M.A.; Dumontet V.; Gallard J.F.; Guillot R.; Leblanc K.; N’nang E.O.; Turpin V.; Kouklovsky C.; Poupon E.; Evanno L.; Vincent G. Nat. Chem. 2017, 9, 793.
|
| [78] |
Li L.; Yuan K.; Jia Q.L.; Jia Y.X. Angew. Chem., Int. Ed. 2019, 58, 6074.
|
| [79] |
Tan D.X.; Zhou J.; Liu C.Y.; Han F.S. Angew. Chem., Int. Ed. 2020, 59, 3834.
|
| [80] |
Yu Y.Y.; Li G.; Jiang L.; Zuo L.S. Angw. Chem., Int. Ed. 2015, 54, 12627.
|
| [1] | Huakun Wang, Xiaolong Ren, Yining Xuan. Study of the Halide Salt Catalyzed [3+2] Cycloaddition of α,β-Epoxy Carboxylate with Isocyanate [J]. Chinese Journal of Organic Chemistry, 2024, 44(1): 251-258. |
| [2] | Mengzhu Li, Boying Meng, Wenjie Lan, Bin Fu. Synthesis of 2,3-Disubstituted Dihydrobenzofurans from o-Quinone Methides and Sulfur Ylides [J]. Chinese Journal of Organic Chemistry, 2024, 44(1): 195-203. |
| [3] | Zuliang Chen, Yingjing Wei, Junliang Zhang. Recent Advances in Cycloaddition Reactions of Donor-Acceptor Aziridines via Carbon-Carbon Bond Cleavage [J]. Chinese Journal of Organic Chemistry, 2023, 43(9): 3078-3088. |
| [4] | Hu Ma, Danfeng Huang, Kehu Wang, Duoduo Tang, Yang Feng, Yuanyuan Reng, Junjiao Wang, Yulai Hu. Synthesis of 3-Trifluoromethylpyrazole Derivatives [J]. Chinese Journal of Organic Chemistry, 2023, 43(9): 3257-3267. |
| [5] | Yi Wang, Jian Zhang, Yangzi Liu, Xiaoyan Luo, Weiping Deng. Palladium-Catalyzed Asymmetric [3+4] Cycloadditions for the Construction of Cyclohepta[b]indoles [J]. Chinese Journal of Organic Chemistry, 2023, 43(8): 2864-2877. |
| [6] | Deliang Kong, Wen Dai, Yiling Zhao, Yilin Chen, Hongping Zhu. Study on Oxidative Cycloaddition Reactions of Amidinatoboryl-aminosilylenes toward Ketone and Diketone Molecules [J]. Chinese Journal of Organic Chemistry, 2023, 43(5): 1843-1851. |
| [7] | Fang Wei, Xin Yu, Qiang Xiao. Advances in C—N3 Retention Reactions Involving Organic Azides [J]. Chinese Journal of Organic Chemistry, 2023, 43(4): 1365-1385. |
| [8] | Chengfu Zeng, Yuan He, Qing Li, Lin Dong. Ir(III)-Catalyzed Novel Three-Component Cascade Trifluoroethoxylation and One-Pot Method to Construct Complex Amide Compounds [J]. Chinese Journal of Organic Chemistry, 2023, 43(3): 1115-1123. |
| [9] | Haiqing Wang, Shuang Yang, Yuchen Zhang, Feng Shi. Advances in Catalytic Asymmetric Reactions Involving o-Hydroxybenzyl Alcohols [J]. Chinese Journal of Organic Chemistry, 2023, 43(3): 974-999. |
| [10] | Chunbo Dai, Siqi Xia, Xiaoyu Chen, Limin Yang. N-Heterocyclic Carbene (NHC)-Catalyzed [4+3] Cycloaddition to Synthesize 4-Aminobenzoheptenolactons [J]. Chinese Journal of Organic Chemistry, 2023, 43(3): 1084-1090. |
| [11] | Yueling Liu, Xinxin Zhong, Ganbing Zhang. Density Functional Theory Study for Exploring the Mechanisms of the [3+2] Cycloaddition Reactions between 1-R-3-Phenylpropylidenecyclopropane (R=Me/H) and Furfural Catalyzed by Pd(0) [J]. Chinese Journal of Organic Chemistry, 2023, 43(2): 660-667. |
| [12] | Junxiu Liang, Yazhou Liu, Amu Wang, Yanchao Wu, Xiaofeng Ma, Huijing Li. Dearomatization of Halonaphthols via an Intermolecular [4+1] Spiroannulation with in situ Formed Aza-ortho-quinone Methides [J]. Chinese Journal of Organic Chemistry, 2023, 43(11): 3888-3899. |
| [13] | Weilu Zhang, Shaowei Chen, Xiao Shen. Nickel-Catalyzed [4+2] Cyclization of Benzosilacyclobutenes and Acylsilanes [J]. Chinese Journal of Organic Chemistry, 2023, 43(10): 3635-3643. |
| [14] | Xiaoting Qin, Ning Zou, Caimei Nong, Dongliang Mo. Recent Advances on the Synthesis of Nine-Membered N-Heterocycles [J]. Chinese Journal of Organic Chemistry, 2023, 43(1): 130-155. |
| [15] | Yun Shi, Ting Xiao, Dong Xia, Wenchao Yang. SCF3 Radical Initiated Cascade Reaction of Unsaturated Hydrocarbon [J]. Chinese Journal of Organic Chemistry, 2022, 42(9): 2715-2727. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||