Chinese Journal of Organic Chemistry ›› 2021, Vol. 41 ›› Issue (4): 1683-1690.DOI: 10.6023/cjoc202009044 Previous Articles Next Articles
Special Issue: 有机光催化虚拟合辑
ARTICLES
收稿日期:2020-09-20
修回日期:2020-11-15
发布日期:2020-12-10
通讯作者:
左伟伟
基金资助:
Shangfei Huo1, Hong Chen1, Weiwei Zuo1,*( )
)
Received:2020-09-20
Revised:2020-11-15
Published:2020-12-10
Contact:
Weiwei Zuo
About author:Supported by:Share
Shangfei Huo, Hong Chen, Weiwei Zuo. Selective Chlorination of Methane Photochemically Mediated by Ferric Chloride at Ambient Temperature[J]. Chinese Journal of Organic Chemistry, 2021, 41(4): 1683-1690.





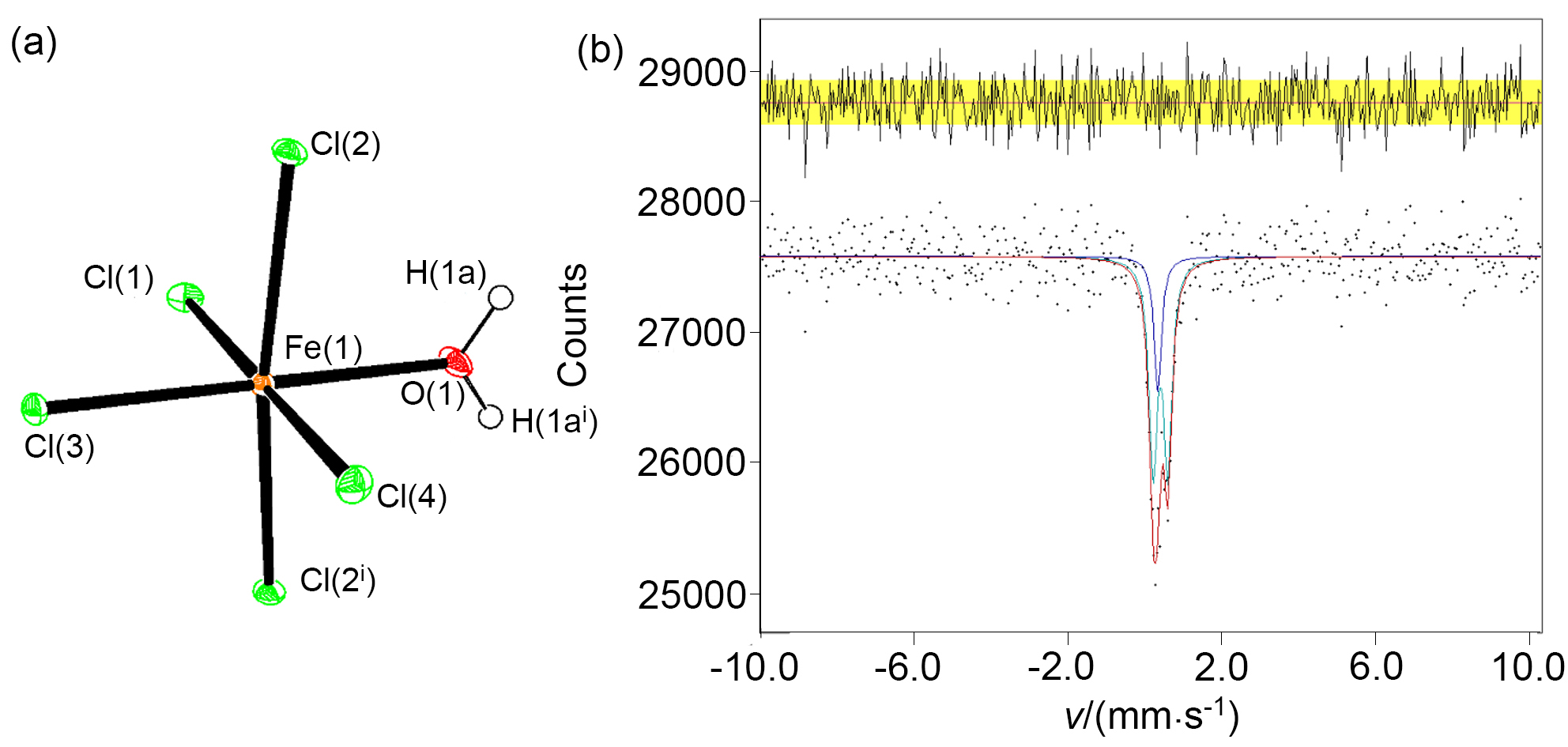
| [1] |
Lamarche-Gagnon, G.; Wadham, J.L.; Sherwood Lollar, B.; Arndt, S.; Fietzek, P.; Beaton, A.D.; Tedstone, A.J.; Telling, J.; Bagshaw, E.A.; Hawkings, J.R.; Kohler, T.J.; Zarsky, J.D.; Mowlem, M.C.; Anesio, A.M.; Stibal, M. Nature 2019, 565,73.
doi: 10.1038/s41586-018-0800-0 pmid: 30602750 |
| [2] |
(a) McFarland, E. Science 2012, 338,340.
pmid: 28150944 |
|
(b) Gunsalus, N.J.; Koppaka, A.; Park, S.H.; Bischof, S.M.; Hashiguchi, B.G.; Periana, R.A. Chem. Rev. 2017, 117,8521.
doi: 10.1021/acs.chemrev.6b00739 pmid: 28150944 |
|
|
(c) Lin, R.; Amrute, A.P.; Perez-Ramirez, J. Chem. Rev. 2017, 117,4182.
doi: 10.1021/acs.chemrev.6b00551 pmid: 28150944 |
|
|
(d) Ravi, M.; Ranocchiari, M.; van Bokhoven, J.A. Angew. Chem., nt. Ed. 2017, 56,16464.
pmid: 28150944 |
|
| [3] |
(a) Fletcher, S.E. M.; Schaefer, H. Science 2019, 364,932.
doi: 10.1126/science.aax1828 pmid: 12037558 |
|
(b) Labinger, J.A.; Bercaw, J.E. Nature 2002, 417,507.
pmid: 12037558 |
|
|
(c) Shilov, A.E.; Shul'pin, G.B. Chem. Rev. 1997, 97,2879.
pmid: 12037558 |
|
|
(d) Cavaliere, V.N.; Wicker, B.F.; Mindiola, D.J. Adv. Organomet. Chem. 2012, 60,1.
pmid: 12037558 |
|
| [4] |
(a) Alvarez-Galvan, M.C.; Mota, N.; Ojeda, M.; Rojas, S.; Navarro, R.M.; Fierro, J.L. G. Catal. Today 2011, 171,15.
|
|
(b) Olsbye, U. Angew. Chem., nt. Ed. 2016, 55,7294.
|
|
| [5] |
(a) Zimmermann, T.; Soorholtz, M.; Bilke, M.; Schuth, F. J. Am. Chem. Soc. 2016, 138,12395.
doi: 10.1021/jacs.6b05167 pmid: 9554841 |
|
(b) Periana, R.A.; Taube, D.J.; Evitt, E.R.; Loffler, D.G.; Wentrcek, P.R.; Voss, G.; Masuda, T. Science 1993, 259,340.
pmid: 9554841 |
|
|
(c) Periana, R.A.; Taube, D.J.; Gamble, S.; Taube, H.; Satoh, T.; Fujii, H. Science 1998, 280,560.
doi: 10.1126/science.280.5363.560 pmid: 9554841 |
|
|
(d) Lin, M.; Sen, A. Nature 1994, 368,613.
pmid: 9554841 |
|
|
(e) Periana, R.A.; Mironov, O.; Taube, D.; Bhalla, G.; Jones, C.J. Science 2003, 301,814.
pmid: 9554841 |
|
|
(f) Gretz, E.; Oliver, T.F.; Sen, A. J. Am. Chem. Soc. 1987, 109,8109.
pmid: 9554841 |
|
|
(g) Vargaftik, M.N.; Stolarov, I.P.; Moiseev, I.I. J. Chem. Soc., hem. Commun. 1990,1049.
pmid: 9554841 |
|
|
(h) Kao, L.C.; Hutson, A.C.; Sen, A. J. Am. Chem. Soc. 1991, 113,700.
pmid: 9554841 |
|
| [6] |
(a) Takanabe, K.; Iglesia, E. Angew. Chem., nt. Ed. 2008, 47,7689.
pmid: 30924338 |
|
(b) Hammond, C.; Conrad, S.; Hermans, I. ChemSusChem 2012, 5,1668.
pmid: 30924338 |
|
|
(c) Lunsford, J.H. Angew. Chem., nt. Ed. Engl. 1995, 34,970.
pmid: 30924338 |
|
|
(d) Meng, L.; Chen, Z.; Ma, Z.; He, S.; Hou, Y.; Li, H.-H.; Yuan, R.; Huang, X.-H.; Wang, X.; Wang, X.; Long, J. Energy Environ. Sci. 2018, 11,294.
pmid: 30924338 |
|
|
(e) Wu, S.; Tan, X.; Lei, J.; Chen, H.; Wang, L.; Zhang, J. J. Am. Chem. Soc. 2019, 141,6592.
pmid: 30924338 |
|
| [7] |
Horn, R.; Schloegl, R. Catal. Lett. 2015, 145,23.
|
| [8] |
(a) Bilke, M.; Losch, P.; Vozniuk, O.; Bodach, A.; Schuth, F. J. Am. Chem. Soc. 2019, 141,11212.
doi: 10.1021/jacs.9b04413 pmid: 17295483 |
|
(b) Olah, G.A.; Gupta, B.; Farina, M.; Felberg, J.D.; Ip, W.M.; Husain, A.; Karpeles, R.; Lammertsma, K.; Melhotra, A.K.; Trivedi, N.J. J. Am. Chem. Soc. 1985, 107,7097.
pmid: 17295483 |
|
|
(c) Zhou, X.P.; Yilmaz, A.; Yilmaz, G.A.; Lorkovic, I.M.; Laverman, L.E.; Weiss, M.; Sherman, J.H.; McFarland, E.W.; Stucky, G.D.; Ford, P.C. Chem. Commun. 2003,2294.
pmid: 17295483 |
|
|
(d) Paunovic, V.; Zichittella, G.; Moser, M.; Amrute, A.P.; Perez-Ramirez, J. Nat. Chem. 2016, 8,803.
doi: 10.1038/nchem.2522 pmid: 17295483 |
|
|
(e) Podkolzin, S.G.; Stangland, E.E.; Jones, M.E.; Peringer, E.; Lercher, J.A. J. Am. Chem. Soc. 2007, 129,2569.
pmid: 17295483 |
|
|
(f) He, J.; Xu, T.; Wang, Z.; Zhang, Q.; Deng, W.; Wang, Y. Angew. Chem., nt. Ed. 2012, 51,2438.
pmid: 17295483 |
|
| [9] |
(a) Li, F.; Yuan, G. Angew. Chem., nt. Ed. 2006, 45,6541.
pmid: 29199824 |
|
(b) Batamack, P.T. D.; Mathew, T.; Prakash, G.K. S. J. Am. Chem. Soc. 2017, 139,18078.
doi: 10.1021/jacs.7b10725 pmid: 29199824 |
|
| [10] |
(a) Paunovic, V.; Lin, R.; Scharfe, M.; Amrute, A.P.; Mitchell, S.; Hauert, R.; Perez-Ramirez, J. Angew. Chem., nt. Ed. 2017, 56,9791.
|
|
(b) Shalygin, A.; Paukshtis, E.; Kovalyov, E.; Bal'Zhinimaev, B. Front. Chem. Sci. Eng. 2013, 7,279.
|
|
| [11] |
(a) Maldotti, A.; Molinari, A.; Amadelli, R. Chem. Rev. 2002, 102,3811.
doi: 10.1021/cr010364p pmid: 12371903 |
|
(b) Wu, W.; Fu, Z.; Wen, X.; Wang, Y.; Zou, S.; Meng, Y.; Liu, Y.; Kirk, S.R.; Yin, D. Appl. Catal. A: Gen. 2014, 469,483.
pmid: 12371903 |
|
| [12] |
Blanksby, S.J.; Ellison, G.B. Acc. Chem. Res. 2003, 36,255.
pmid: 12693923 |
| [13] |
(a) Minisci, F.; Fontana, F. Tetrahedron Lett. 1994, 35,1427.
pmid: 17737550 |
|
(b) Kochi, J.K. Science 1967, 155,415.
doi: 10.1126/science.155.3761.415 pmid: 17737550 |
|
| [14] |
Bach, R.D.; Shobe, D.S.; Schlegel, H.B.; Nagel, C.J. J. Phys. Chem. 1996, 100,8770.
|
| [15] |
Chang, P. K. A.J.-H. C. J. Org. Chem. 1971, 36,3138.
|
| [16] |
Miller, J. WO 9959946, 1999.
|
| [17] |
(a) Roberts, B.P. Chem. Soc. Rev. 1999, 28,25.
pmid: 28636596 |
|
(b) Le, C.; Liang, Y.; Evans, R.W.; Li, X.; Mac-Millan, D.W. C. Nature 2017, 547,79.
pmid: 28636596 |
|
| [18] |
(a) Sorokin, A.B.; Kudrik, E.V.; Alvarez, L.X.; Afanasiev, P.; Millet, J.M. M.; Bouchu, D. Catal. Today 2010, 157,149.
pmid: 17676842 |
|
(b) An, Z.-J.; Pan, X.-L.; Liu, X.-M.; Han, X.-W.; Bao, X.-H. J. Am. Chem. Soc. 2006, 128,16028.
pmid: 17676842 |
|
|
(c) Bar-Nahum, I.; Khenkin, A.M.; Neumann, R. J. Am. Chem. Soc. 2004, 126,10236.
doi: 10.1021/ja0493547 pmid: 17676842 |
|
|
(d) Muehlhofer, M.; Strassner, T.; Herrmann, W.A. Angew. Chem., nt. Ed. 2002, 41,1745.
pmid: 17676842 |
|
|
(e) Kirillova, M.V.; Kuznetsov, M.L.; Reis, P.M.; da Silva, J.A. L.; da Silva, J. J., R.F.; Pombeiro, A.J. L. J. Am. Chem. Soc. 2007, 129,10531.
doi: 10.1021/ja072531u pmid: 17676842 |
|
| [19] |
(a) Lange, J.-P. ChemSusChem 2017, 10,245.
doi: 10.1002/cssc.201600855 pmid: 27763723 |
|
(b) Lange, J.P. CATTECH 2001, 5,82.
pmid: 27763723 |
|
| [20] |
Lange, J.-P. Catal. Sci. Technol. 2016, 6,4759.
|
| [21] |
Lange, J.-P.; Sushkevich, V.L.; Knorpp, A.J.; van Bokhoven, J.A. Ind. Eng. Chem. Res. 2019, 58,8674.
|
| [22] |
Knuuttila, P.T.; Jokinen, S.S.; Judin, V.O.; Vuorisalo, J.T.; Salanne, S.S. EP 0493023, 1992.
|
| [23] |
Horvath, I.T.; Pa, N.H.; Summit, J.M. M.; Cook, R.A. US 5354916, 1994.
|
| [24] |
Mizuta, S.; Kondo, W.; Fujii, K.; Iida, H.; Isshiki, S.; Noguchi, H.; Kikuchi, T.; Sue, H.; Sakai, K. Ind. Eng. Chem. Res. 1991, 30,1601.
|
| [1] | Qinggang Mei, Qinghan Li. Recent Progress of Visible Light-Induced the Synthesis of C(3) (Hetero)arylthio Indole Compounds [J]. Chinese Journal of Organic Chemistry, 2024, 44(2): 398-408. |
| [2] | Yanshuo Zhu, Hongyan Wang, Penghua Shu, Ke'na Zhang, Qilin Wang. Recent Advances on Alkoxy Radicals-Mediated C(sp3)—H Bond Functionalization via 1,5-Hydrogen Atom Transfer [J]. Chinese Journal of Organic Chemistry, 2024, 44(1): 1-17. |
| [3] | Hongqiong Zhao, Miao Yu, Dongxue Song, Qi Jia, Yingjie Liu, Yubin Ji, Ying Xu. Progress on Decarboxylation and Hydroxylation of Carboxylic Acids [J]. Chinese Journal of Organic Chemistry, 2024, 44(1): 70-84. |
| [4] | Yukun Jin, Baoyi Ren, Fushun Liang. Visible Light-Mediated Selective C—F Bond Cleavage of Trifluoromethyl Groups and Its Application in Synthesizing gem-Difluoro-Containing Compounds [J]. Chinese Journal of Organic Chemistry, 2024, 44(1): 85-110. |
| [5] | Xiaona Yang, Hongyu Guo, Rong Zhou. Progress in Visible-Light Promoted Transformations of Organosilicon Compounds [J]. Chinese Journal of Organic Chemistry, 2023, 43(8): 2720-2742. |
| [6] | Jiaxia Pu, Xiaoying Jia, Lirong Han, Qinghan Li. Research Progress of Visible Light Promoted C—N Bond Fracture to Construct C—C Bond [J]. Chinese Journal of Organic Chemistry, 2023, 43(8): 2591-2613. |
| [7] | Lingna Wang, Xiaoqing Liu, Gang Lin, Hongying Jin, Minjun Jiao, Xuefen Liu, Shuping Luo. Photocatalytic Activation of C(sp3)—H Bonds to Form C—S Bonds Catalyzed by (Oxybis(4,1-phenylene))bis(phenylmethanone) [J]. Chinese Journal of Organic Chemistry, 2023, 43(8): 2848-2854. |
| [8] | Yu Zhao, Kai Zhang, Yubin Bai, Yantu Zhang, Shihui Shi. A Metal-Free Photocatalytic Hydrosilylation of Alkenes Using Bromide Salt as a Hydrogen Atom Transfer Reagent [J]. Chinese Journal of Organic Chemistry, 2023, 43(8): 2837-2847. |
| [9] | Yingjie Liu, Gangqing Shi, Ge Chou, Xin Zhang, Dongxue Song, Ning Chen, Miao Yu, Ying Xu. Progress of α-Position Functionalization of Ethers under Photo/Electrocatalysis [J]. Chinese Journal of Organic Chemistry, 2023, 43(8): 2664-2681. |
| [10] | Sifan Dong, Haolong Li, Yuan Qin, Shiming Fan, Shouxin Liu. Research Progress of Amino Acids as Transient Directing Groups in C—H Bond Activation Reactions [J]. Chinese Journal of Organic Chemistry, 2023, 43(7): 2351-2367. |
| [11] | Yaxin Liu, Yu Zhang, Shuping Luo. Design and Synthesis of Thermal Delayed Fluorescence (TADF) Photocatalyst and Its Photocatalytic Dehalogenation Performance [J]. Chinese Journal of Organic Chemistry, 2023, 43(7): 2476-2483. |
| [12] | Ning Chen, Chengdong Zhang, Peng Li, Ge Qiu, Yinjie Liu, Tianlei Zhang. Research Progress in Synthesis of Spirocyclic Compounds Driven by Photo/Electrochemistry [J]. Chinese Journal of Organic Chemistry, 2023, 43(7): 2293-2303. |
| [13] | Zhongrong Xu, Jieping Wan, Yunyun Liu. Transition Metal-Free C—H Thiocyanation and Selenocyanation Based on Thermochemical, Photocatalytic and Electrochemical Process [J]. Chinese Journal of Organic Chemistry, 2023, 43(7): 2425-2446. |
| [14] | Xiaojing Hu, Feixiang Guo, Runqing Zhu, Bingqi Zhou, Tao Zhang, Lizhen Fang. Synthesis of p-Alkoxy Phenol and Its Application after Dearomatization [J]. Chinese Journal of Organic Chemistry, 2023, 43(6): 2239-2244. |
| [15] | Shenhao Chen, Song Zou, Chanjuan Xi. Photocatalyzed 2∶2 Coupling of Styrene and BrCF2CO2Me: A Facile Synthesis of Bis-difluoroacetylated Hexestrol Derivatives [J]. Chinese Journal of Organic Chemistry, 2023, 43(3): 1157-1167. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||