Chinese Journal of Organic Chemistry ›› 2021, Vol. 41 ›› Issue (10): 3948-3964.DOI: 10.6023/cjoc202104032 Previous Articles Next Articles
Special Issue: 南开大学化学学科创立100周年
REVIEWS
收稿日期:2021-04-16
修回日期:2021-05-12
发布日期:2021-06-17
通讯作者:
渠瑾
基金资助:Received:2021-04-16
Revised:2021-05-12
Published:2021-06-17
Contact:
Jin Qu
Supported by:Share
Feifan Li, Jin Qu. Synthesis of Aryl or Heteroaryl C-Nucleosides by Direct Coupling of a Carbohydrate Moiety with a Preformed Aglycon Unit[J]. Chinese Journal of Organic Chemistry, 2021, 41(10): 3948-3964.
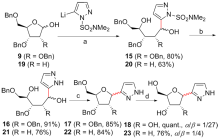
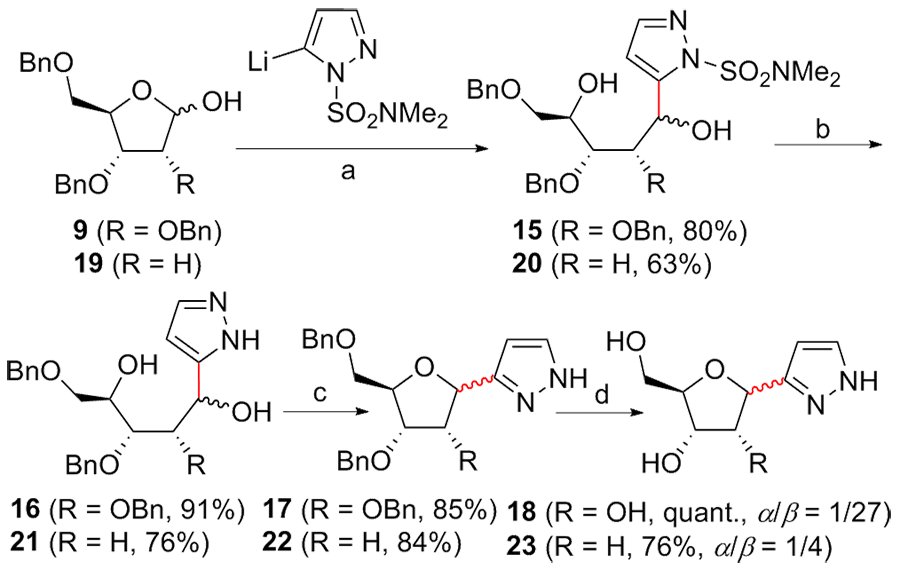
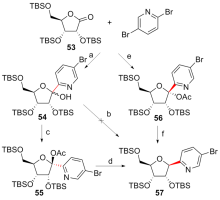
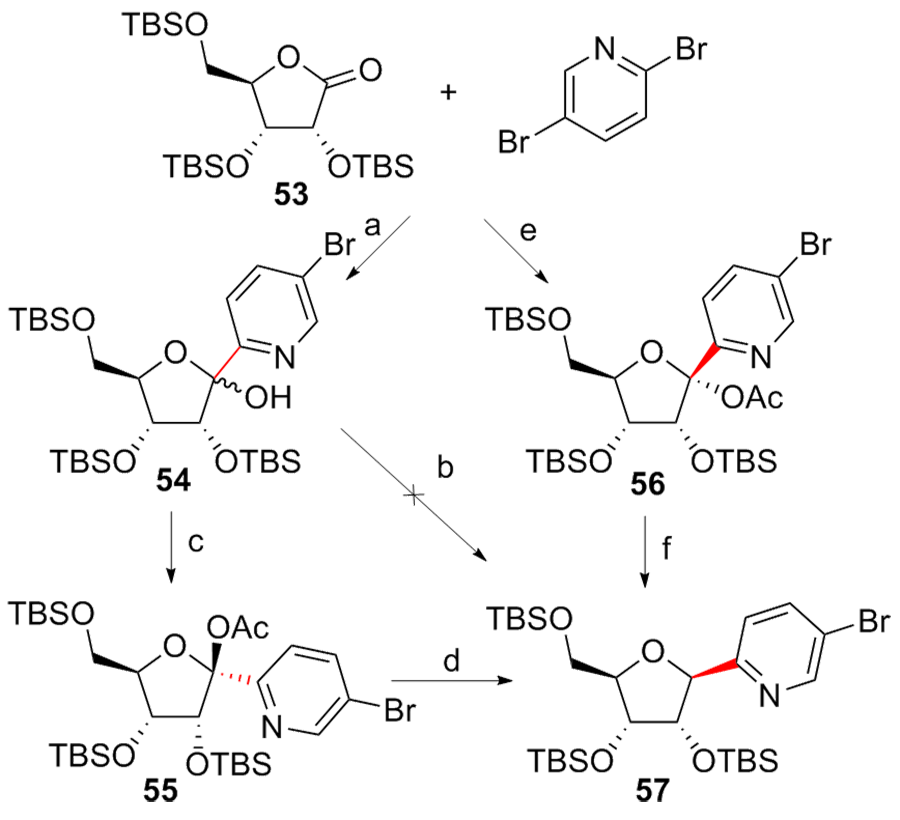
| [1] |
Štambaský, J.; Hocek, M.; Kočovský, P. Chem. Rev. 2009, 109, 6729.
doi: 10.1021/cr9002165 |
| [2] |
De Clercq, E. J. Med. Chem. 2016, 59, 2301.
doi: 10.1021/acs.jmedchem.5b01157 pmid: 26513594 |
| [3] |
Townsend, L. B. Chemistry of Nucleosides and Nucleotides, Plenum Press, New York, 1994, pp. 421-535.
|
| [4] |
(a) Arnez, J. G.; Steitz, T. A. Biochemistry 1994, 33, 7560.
pmid: 7688564 |
|
(b) Davis, D. R.; Veltri, C. A.; Nielsen, L. J. Biomol. Struct. Dyn. 1998, 15, 1121.
pmid: 7688564 |
|
|
(c) Harrington, K. M.; Nazarenko, I. A.; Dix, D. B.; Thompson, R. C.; Uhlenbeck, O. C. Biochemistry 1993, 32, 7617.
pmid: 7688564 |
|
| [5] |
Nishimura, H.; Mayama, M.; Komatsu, Y.; Shimaoka, N.; Tanaka, Y.; Kato, H. J. Antibiot. 1964, 17, 148.
|
| [6] |
Koyama, G.; Maeda, K.; Umezawa, H.; Iitaka, Y. Terahedron Lett. 1966, 7, 597.
doi: 10.1016/S0040-4039(01)99671-6 |
| [7] |
Kusakabe, Y.; Nagatsu, J.; Shibuya, M.; Kawaguchi, O.; Hirose, C.; Shirato, S. J. Antibiot. 1972, 25, 44.
pmid: 5010645 |
| [8] |
Gutowski, G. E.; Sweeney, M. J.; DeLong, D. C.; Hamill, R. L.; Gerzon, K.; Dyke, R. W. Ann. N. Y. Acad. Sci. 1975, 255, 544.
doi: 10.1111/nyas.1975.255.issue-1 |
| [9] |
(a) Robins, R. K.; Srivastava, P. C.; Narayanan, V. L.; Plowman, J.; Paull, K. D. J. Med. Chem. 1982, 25, 107.
pmid: 6130768 |
|
(b) Cooney, D. A.; Jayaram, H. N.; Gebeyehu, G.; Betts, C. R.; Kelley, J. A.; Marquez, V. E.; Johns, D. G. Biochem. Pharmacol. 1982, 31, 2133.
pmid: 6130768 |
|
|
(c) Jayaram, H. N.; Cooney, D. A.; Glazer, R. I.; Dion, R. L.; Johns, D. G. Biochem. Pharmacol. 1982, 31, 2557.
pmid: 6130768 |
|
|
(d) Jayaram, H. N.; Dion, R. L.; Glazer, R. I.; Johns, D. G.; Robins, R. K.; Srivastava, P. C.; Cooney, D. A. Biochem. Pharmacol. 1982, 31, 2371.
pmid: 6130768 |
|
|
(e) Jayaram, H. N.; Smith, A. L.; Glazer, R. I.; Johns, D. G.; Cooney, D. A. Biochem. Pharmacol. 1982, 31, 3839.
pmid: 6130768 |
|
| [10] |
Li, G.; De Clercq, E. Nat. Rev. Drug Discovery 2020, 19, 149.
doi: 10.1038/d41573-020-00016-0 |
| [11] |
Warren, T. K.; Jordan, R.; Lo, M. K.; Ray, A. S.; Mackman, R. L.; Soloveva, V.; Siegel, D.; Perron, M.; Bannister, R.; Hui, H. C.; Larson, N.; Strickley, R.; Wells, J.; Stuthman, K. S.; Van Tongeren, S. A.; Garza, N. L.; Donnelly, G.; Shurtleff, A. C.; Retterer, C. J.; Gharaibeh, D.; Zamani, R.; Kenny, T.; Eaton, B. P.; Grimes, E.; Welch, L. S.; Gomba, L.; Wilhelmsen, C. L.; Nichols, D. K.; Nuss, J. E.; Nagle, E. R.; Kugelman, J. R.; Palacios, G.; Doerffler, E.; Neville, S.; Carra, E.; Clarke, M. O.; Zhang, L.; Lew, W.; Ross, B.; Wang, Q.; Chun, K.; Wolfe, L.; Babusis, D.; Park, Y.; Stray, K. M.; Trancheva, I.; Feng, J. Y.; Barauskas, O.; Xu, Y.; Wong, P.; Braun, M. R.; Flint, M.; McMullan, L. K.; Chen, S.-S.; Fearns, R.; Swaminathan, S.; Mayers, D. L.; Spiropoulou, C. F.; Lee, W. A.; Nichol, S. T.; Cihlar, T.; Bavari, S. Nature 2016, 531, 381.
doi: 10.1038/nature17180 |
| [12] |
(a) Warren, T. K.; Wells, J.; Panchal, R. G.; Stuthman, K. S.; Garza, N. L.; Van Tongeren, S. A.; Dong, L.; Retterer, C. J.; Eaton, B. P.; Pegoraro, G.; Honnold, S.; Bantia, S.; Kotian, P.; Chen, X.; Taubenheim, B. R.; Welch, L. S.; Minning, D. M.; Babu, Y. S.; Sheridan, W. P.; Bavari, S. Nature 2014, 508, 402.
doi: 10.1038/nature13027 |
|
(b) Taylor, R.; Kotian, P.; Warren, T.; Panchal, R.; Bavari, S.; Julander, J.; Dobo, S.; Rose, A.; El-Kattan, Y.; Taubenheim, B.; Babu, Y.; Sheridan, W. P. J. Infect. Public Heal. 2016, 9, 220.
|
|
| [13] |
Julander, J. G.; Bantia, S.; Taubenheim, B. R.; Minning, D. M.; Kotian, P.; Morrey, J. D.; Smee, D. F.; Sheridan, W. P.; Babu, Y. S. Antimicrob. Agents Chemother. 2014, 58, 6607.
doi: 10.1128/AAC.03368-14 pmid: 25155605 |
| [14] |
Wang, G.; Wan, J.; Hu, Y.; Wu, X.; Prhavc, M.; Dyatkina, N.; Rajwanshi, V. K.; Smith, D. B.; Jekle, A.; Kinkade, A.; Symons, J. A.; Jin, Z.; Deval, J.; Zhang, Q.; Tam, Y.; Chanda, S.; Blatt, L.; Beigelman, L. J. Med. Chem. 2016, 59, 4611.
doi: 10.1021/acs.jmedchem.5b01933 |
| [15] |
Wu, Q.; Simons, C. Synthesis 2004, 1533.
|
| [16] |
Adamo, M. F. A.; Pergoli, R. Curr. Org. Chem. 2008, 12, 1544.
doi: 10.2174/138527208786786318 |
| [17] |
Brown, D. M.; Ogden, R. C. J. Chem. Soc., Perkin Trans. 1 1981, 723.
|
| [18] |
Krohn, K.; Heins, H.; Wielckens, K. J. Med. Chem. 1992, 35, 511.
pmid: 1738143 |
| [19] |
Harusawa, S.; Matsuda, C.; Araki, L.; Kurihara, T. Synthesis 2006, 793.
|
| [20] |
Solomon, M. S.; Hopkins, P. B. Tetrahedron Lett. 1991, 32, 3297.
doi: 10.1016/S0040-4039(00)92690-X |
| [21] |
Spadafora, M.; Burger, A.; Benhida, R. Synlett 2008, 1225.
|
| [22] |
Peyron, C.; Benhida, R. Synlett 2009, 472.
|
| [23] |
Piccirilli, J. A.; Krauch, T.; Macpherson, L. J.; Benner, S. A. Helv. Chim. Acta 1991, 74, 397.
doi: 10.1002/(ISSN)1522-2675 |
| [24] |
Kim, T. W.; Kool, E. T. Org. Lett. 2004, 6, 3949.
doi: 10.1021/ol048487u |
| [25] |
van Rijssel, E. R.; van Delft, P.; Lodder, G.; Overkleeft, H. S.; van der Marel, G. A.; Filippov, D. V.; Codée, J. D. C. Angew. Chem., Int. Ed. 2014, 53, 10381.
doi: 10.1002/anie.201405477 |
| [26] |
van Rijssel, E. R.; van Delft, P.; van Marle, D. V.; Bijvoets, S. M.; Lodder, G.; Overkleeft, H. S.; van der Marel, G. A.; Filippov, D. V.; Codée, J. D. C. J. Org. Chem. 2015, 80, 4553.
doi: 10.1021/acs.joc.5b00419 pmid: 25826382 |
| [27] |
Larsen, C. H.; Ridgway, B. H.; Shaw, J. T.; Smith, D. M.; Woerpel, K. A. J. Am. Chem. Soc. 2005, 127, 10879.
doi: 10.1021/ja0524043 |
| [28] |
Štefko, M.; Slavětínská, L.; Klepetářová, B.; Hocek, M. J. Org. Chem. 2011, 76, 6619.
doi: 10.1021/jo200949c |
| [29] |
Asbun, W.; Binkley, S. B. J. Org. Chem. 1968, 31, 140.
|
| [30] |
Enders, D.; Hieronymi, A.; Ridder, A. Synlett 2005, 2391.
|
| [31] |
Guianvarc'h, D.; Fourrey, J. L.; Huu Dau, M. T.; Guérineau, V.; Benhida, R. J. Org. Chem. 2002, 67, 3724.
pmid: 12027686 |
| [32] |
Cho, A.; Saunders, O. L.; Butler, T.; Zhang, L.; Xu, J.; Vela, J. E.; Feng, J. Y.; Ray, A. S.; Kim, C. U. Bioorg. Med. Chem. Lett. 2012, 22, 2705.
doi: 10.1016/j.bmcl.2012.02.105 |
| [33] |
Metobo, S. E.; Xu, J.; Saunders, O. L.; Butler, T.; Aktoudianakis, E.; Cho, A.; Kim, C. U. Tetrahedron Lett. 2012, 53, 484.
doi: 10.1016/j.tetlet.2011.11.055 |
| [34] |
Chaudhuri, N. C.; Kool, E. T. Tetrahedron Lett. 1995, 36, 1795.
doi: 10.1016/0040-4039(95)00145-3 |
| [35] |
Shapiro, R.; Chambers, R. W. J. Am. Chem. Soc. 1961, 83, 3920.
doi: 10.1021/ja01479a057 |
| [36] |
Schweitzer, B. A.; Kool, E. T. J. Org. Chem. 1994, 59, 7238.
pmid: 20882116 |
| [37] |
Hocek, M.; Pohl, R.; Klepetářová, B. Eur. J. Org. Chem. 2005, 2005, 4525.
doi: 10.1002/(ISSN)1099-0690 |
| [38] |
Joubert, N.; Urban, M.; Pohl, R.; Hocek, M. Synthesis 2008, 1918.
|
| [39] |
Weinberger, M.; Wagenknecht, H. A. Synthesis 2012, 44, 648.
|
| [40] |
Jiang, Y. L.; Stivers, J. T. Tetrahedron Lett. 2003, 44, 4051.
doi: 10.1016/S0040-4039(03)00848-7 |
| [41] |
Nicolas, L.; Angibaud, P.; Stansfield, I.; Meerpoel, L.; Reymond, S.; Cossy, J. Tetrahedron Lett. 2014, 55, 849.
doi: 10.1016/j.tetlet.2013.12.035 |
| [42] |
Beuck, C.; Singh, I.; Bhattacharya, A.; Hecker, W.; Parmar, V. S.; Seitz, O.; Weinhold, E. Angew. Chem., Int. Ed. 2003, 42, 3958.
doi: 10.1002/(ISSN)1521-3773 |
| [43] |
Ren, R. X.-F.; Chaudhuri, N. C.; Paris, P. L.; Rumney, S.; Kool, E. T. J. Am. Chem. Soc. 1996, 118, 7671.
doi: 10.1021/ja9612763 |
| [44] |
Hainke, S.; Singh, I.; Hemmings, J.; Seitz, O. J. Org. Chem. 2007, 72, 8811.
pmid: 17941674 |
| [45] |
Singh, I.; Seitz, O. Org. Lett. 2006, 8, 4319.
doi: 10.1021/ol061701p |
| [46] |
Hacksell, U.; Daves, G. D. J. Org. Chem. 1983, 48, 2870.
doi: 10.1021/jo00165a017 |
| [47] |
Cheng, J. C. Y.; Hacksell, U.; Daves, G. D. J. Org. Chem. 1986, 51, 3093.
doi: 10.1021/jo00366a003 |
| [48] |
Zhang, H. C.; Daves, G. D. J. Org. Chem. 1992, 57, 4690.
doi: 10.1021/jo00043a029 |
| [49] |
Raboisson, P.; Baurand, A.; Cazenave, J. P.; Gachet, C.; Schultz, D.; Spiess, B.; Bourguignon, J. J. J. Org. Chem. 2002, 67, 8063.
pmid: 12423133 |
| [50] |
Lefoix, M.; Mathis, G.; Kleinmann, T.; Truffert, J. C.; Asseline, U. J. Org. Chem. 2014, 79, 3221.
doi: 10.1021/jo5000253 |
| [51] |
Joubert, N.; Pohl, R.; Klepetářová, B.; Hocek, M. J. Org. Chem. 2007, 72, 6797.
doi: 10.1021/jo0709504 |
| [52] |
Chapuis, H.; Kubelka, T.; Joubert, N.; Pohl, R.; Hocek, M. Eur. J. Org. Chem. 2012, 1759.
|
| [53] |
Temburnikar, K.; Brace, K.; Seley-Radtke, K. L. J. Org. Chem. 2013, 78, 7305.
doi: 10.1021/jo400913k pmid: 23806030 |
| [54] |
Gong, H.; Gagné, M. R. J. Am. Chem. Soc. 2008, 130, 12177.
doi: 10.1021/ja8041564 |
| [55] |
Nicolas, L.; Izquierdo, E.; Angibaud, P.; Stansfield, I.; Meerpoel, L.; Reymond, S.; Cossy, J. J. Org. Chem. 2013, 78, 11807.
doi: 10.1021/jo401845q pmid: 24127819 |
| [56] |
Adak, L.; Kawamura, S.; Toma, G.; Takenaka, T.; Isozaki, K.; Takaya, H.; Orita, A.; Li, H. C.; Shing, T. K. M.; Nakamura, M. J. Am. Chem. Soc. 2017, 139, 10693.
doi: 10.1021/jacs.7b03867 |
| [57] |
Wang, Q.; An, S.; Deng, Z.; Zhu, W.; Huang, Z.; He, G.; Chen, G. Nat. Catal. 2019, 2, 793.
doi: 10.1038/s41929-019-0324-5 |
| [58] |
Ma, Y.; Liu, S.; Xi, Y.; Li, H.; Yang, K.; Cheng, Z.; Wang, W.; Zhang, Y. Chem. Commun. 2019, 55, 14657.
doi: 10.1039/C9CC07184A |
| [59] |
Mukaiyama, T.; Matsubara, K.; Hora, M. Synthesis 1994, 1368.
|
| [60] |
Shirakawa, S.; Kobayashi, S. Org. Lett. 2007, 9, 311.
pmid: 17217292 |
| [61] |
Sokolova, T. N.; Yartseva, I. V.; Preobrazhenskaya, M. N. Carbohydr. Res. 1981, 93, 19.
doi: 10.1016/S0008-6215(00)80749-1 |
| [62] |
Hainke, S.; Arndt, S.; Seitz, O. Org. Biomol. Chem. 2005, 3, 4233.
pmid: 16294252 |
| [63] |
Bárta, J.; Pohl, R.; Klepetářová, B.; Ernsting, N. P.; Hocek, M. J. Org. Chem. 2008, 73, 3798.
doi: 10.1021/jo800177y pmid: 18416574 |
| [64] |
Macdonald, S. J. F.; Huizinga, W. B.; McKenzie, T. C. J. Org. Chem. 1988, 53, 3371.
doi: 10.1021/jo00249a051 |
| [65] |
Yokoyama, M.; Nomura, M.; Togo, H.; Seki, H. J. Chem. Soc., erkin Trans. 1 1996, 2145.
|
| [66] |
Girgis, N. S.; Michael, M. A.; Smee, D. F.; Alaghamandan, H. A.; Robins, R. K.; Cottam, H. B. J. Med. Chem. 1990, 33, 2750.
pmid: 2170645 |
| [67] |
Liu, M. C.; Luo, M. Z.; Mozdziesz, D. E.; Sartorelli, A. C. Nucleosides Nucleotides Nucleic Acids 2005, 24, 45.
doi: 10.1081/NCN-46784 |
| [68] |
Shin, D.; Sinkeldam, R. W.; Tor, Y. J. Am. Chem. Soc. 2011, 133, 14912.
doi: 10.1021/ja206095a |
| [69] |
Spadafora, M.; Mehiri, M.; Burger, A.; Benhida, R. Tetrahedron Lett. 2008, 49, 3967.
doi: 10.1016/j.tetlet.2008.04.105 |
| [70] |
Marzag, H.; Zerhouni, M.; Tachallait, H.; Demange, L.; Robert, G.; Bougrin, K.; Auberger, P.; Benhida, R. Bioorg. Med. Chem. 2018, 28, 1931.
doi: 10.1016/j.bmcl.2018.03.063 |
| [71] |
Tachallait, H.; Filho, M. S.; Marzag, H.; Bougrin, K.; Demange, L.; Martin, A. R.; Benhida, R. New J. Chem. 2019, 43, 5551.
doi: 10.1039/c8nj06300a |
| [72] |
Hungerford, N. L.; Armitt, D. J.; Banwell, M. G. Synthesis 2003, 1837.
|
| [1] | Wei Xu, Hongbin Zhai, Bin Cheng, Taimin Wang. Visible Light-Induced Pd-Catalyzed Heck Reactions [J]. Chinese Journal of Organic Chemistry, 2023, 43(9): 3035-3054. |
| [2] | Xuqun Zeng, Qian Zhang, Xufeng Wu, Jingfeng Zhang, Xinwei Zhang, Xiaolei Huang. Nickel-Catalyzed Heck Reaction of Cycloalkenes with Inert C—O Bonds of Aryl Carbonates and Aryl Sulfamates [J]. Chinese Journal of Organic Chemistry, 2022, 42(9): 2981-2987. |
| [3] | Xiao Xiao, Jianchao Liu. Progress in the Synthesis of C(sp2)—C(sp3) Bond by Reductive Heck Reactions of Alkenes [J]. Chinese Journal of Organic Chemistry, 2021, 41(9): 3349-3365. |
| [4] | Lingxiang Lian, Jingyi Yin, Caixia Lin, Keyin Ye, Yaofeng Yuan. Phosphine Oxide-Directed Palladium-Catalyzed Heck-Type Functionalization of o-Carboranes [J]. Chinese Journal of Organic Chemistry, 2021, 41(8): 3249-3255. |
| [5] | Junwei Zhang, Hao Wu, Weixin Zhang, Liming Wang, Ying Jin. Enantioselective Friedel-Crafts Reaction of Indoles with Isatins Catalyzed by Cinchona Alkaloid Silyl Ether Derivative [J]. Chinese Journal of Organic Chemistry, 2021, 41(3): 1187-1192. |
| [6] | Zhiyu Hu, Guofang Jiang, Zhiqiang Zhu, Bozhen Gong, Zongbo Xie, Zhanggao Le. One-Pot Domino Henry-Friedel-Crafts Alkylation Reaction in Deep Eutectic Solvent [J]. Chinese Journal of Organic Chemistry, 2021, 41(1): 325-332. |
| [7] | Zeng Hongyun, Zhang Jun'gan, Hong Wei. New Method for the Synthesis of 2,5-Diaryl Substituted Thiazoles [J]. Chinese Journal of Organic Chemistry, 2020, 40(8): 2535-2542. |
| [8] | Han Jilai, Tang Meilin, Sun Xun. Study on the Total Synthesis of Resveratrol Dimers Quadrangularin A and Pallidol [J]. Chinese Journal of Organic Chemistry, 2020, 40(6): 1571-1577. |
| [9] | Huang Jin, Fu Ronghui, Jing Linhai, Qin Dabin, Huang Kun, Wang Wei. A Convenient Access to 3-Substituted Benzofuran Derivatives via Palladium Nanoparticles-Catalyzed Intramolecular Heck Reaction [J]. Chin. J. Org. Chem., 2019, 39(2): 456-462. |
| [10] | Wang Liming, Lin Geng, Zhao Meijun, Liu Dongyao, Jin Ying. Chiral Urea Derivatives Organocatalyzed Asymmetric aza-Friedel-Crafts Reaction of α-Naphthol with N-Tosyl Aldimines [J]. Chin. J. Org. Chem., 2018, 38(3): 642-647. |
| [11] | Sokolov Viacheslav I.. Synthesis and Application of N-Cyclopalladated Ferrocene Derivatives [J]. Chin. J. Org. Chem., 2018, 38(1): 75-85. |
| [12] | Zhang Zhiguo, Zheng Dan, Ma Na'na, Bi Jingjing. Brønsted Acid-Promoted the Synthesis of Furo[2,3-b]quinolines [J]. Chin. J. Org. Chem., 2017, 37(7): 1824-1829. |
| [13] | Dong Xu, Hou Yongzheng, Meng Fanwei, Liu Hongbo, Liu Hui. Recent Advances on Alkyl-Heck Reaction [J]. Chin. J. Org. Chem., 2017, 37(5): 1088-1098. |
| [14] | Nie Biao, Jin Chuanfei, Zhong Wenhe, Ren Qingyun, Zhang Yingjun, Zhang Ji. Application and Recent Progress of Phosphoramidate Prodrugs Strategies and ProTide Technology in Drug Discovery [J]. Chin. J. Org. Chem., 2017, 37(11): 2818-2840. |
| [15] | Song Ran, Yuan Binhua, Sun Xingwen, Lin Guoqiang. A Convenient Access to 1-Aminoindans via Intramolecular Heck Reaction [J]. Chin. J. Org. Chem., 2016, 36(6): 1308-1315. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
