Chinese Journal of Organic Chemistry ›› 2022, Vol. 42 ›› Issue (3): 884-890.DOI: 10.6023/cjoc202109012 Previous Articles Next Articles
ARTICLES
收稿日期:2021-09-06
修回日期:2021-10-11
发布日期:2021-11-03
通讯作者:
沈晓宝
基金资助:
Fufang Wu, Xuejian Li, Hao Jia, Xuanzhen Han, Xiaobao Shen( )
)
Received:2021-09-06
Revised:2021-10-11
Published:2021-11-03
Contact:
Xiaobao Shen
Supported by:Share
Fufang Wu, Xuejian Li, Hao Jia, Xuanzhen Han, Xiaobao Shen. Iodine(III)-Promoted Oxidative Cross-Coupling Reactions of C—H Bonds via a Free Radical Process[J]. Chinese Journal of Organic Chemistry, 2022, 42(3): 884-890.
| Entry | Oxidant (equiv.) | Time/h | Yieldb/% |
|---|---|---|---|
| 1 | TBHP (2) | 2 | N.D. |
| 2 | H2O2 (2) | 2 | N.D. |
| 3 | PhI(OCOCF3)2 (2) | 2 | Trace |
| 4 | PhI(OAc)2 (2) | 2 | 85 |
| 5 | PhI(OAc)2 (2) | 4 | 86 |
| 6 | PhI(OAc)2 (2) | 6 | 83 |
| 7 | PhI(OAc)2 (2) | 8 | 85 |
| 8 | PhI(OAc)2 (2) | 12 | 82 |
| 9 | PhI(OAc)2 (0.1) | 2 | 37 |
| 10 | PhI(OAc)2 (0.5) | 2 | 53 |
| 11 | PhI(OAc)2 (1.0) | 2 | 72 |
| 12 | PhI(OAc)2 (1.2) | 2 | 86 |
| 13 | PhI(OAc)2 (1.5) | 2 | 84 |
| Entry | Oxidant (equiv.) | Time/h | Yieldb/% |
|---|---|---|---|
| 1 | TBHP (2) | 2 | N.D. |
| 2 | H2O2 (2) | 2 | N.D. |
| 3 | PhI(OCOCF3)2 (2) | 2 | Trace |
| 4 | PhI(OAc)2 (2) | 2 | 85 |
| 5 | PhI(OAc)2 (2) | 4 | 86 |
| 6 | PhI(OAc)2 (2) | 6 | 83 |
| 7 | PhI(OAc)2 (2) | 8 | 85 |
| 8 | PhI(OAc)2 (2) | 12 | 82 |
| 9 | PhI(OAc)2 (0.1) | 2 | 37 |
| 10 | PhI(OAc)2 (0.5) | 2 | 53 |
| 11 | PhI(OAc)2 (1.0) | 2 | 72 |
| 12 | PhI(OAc)2 (1.2) | 2 | 86 |
| 13 | PhI(OAc)2 (1.5) | 2 | 84 |
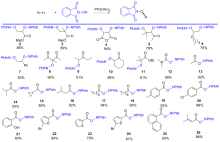
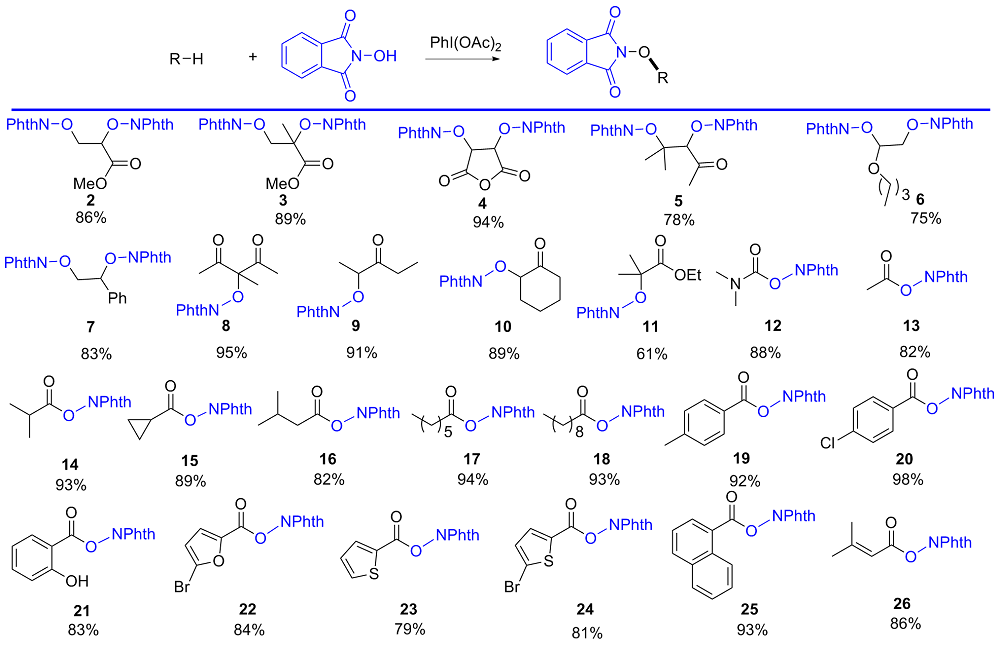
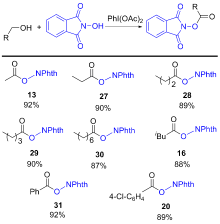

| Substrate | Product | Yieldc | Yieldd/% | ||
|---|---|---|---|---|---|
| TEMPO | BHT | DPPH | |||
| Methyl methacrylate | 3 | 82 | Trace | Trace | Trace |
| 3-Methyl-2,4-pentanedione | 8 | 89 | 15 | 14 | Trace |
| 4-Chlorobenzaldehyde | 20 | 90 | 17 | 18 | Trace |
| C2H5OH | 13 | 86 | Trace | Trace | Trace |
| Substrate | Product | Yieldc | Yieldd/% | ||
|---|---|---|---|---|---|
| TEMPO | BHT | DPPH | |||
| Methyl methacrylate | 3 | 82 | Trace | Trace | Trace |
| 3-Methyl-2,4-pentanedione | 8 | 89 | 15 | 14 | Trace |
| 4-Chlorobenzaldehyde | 20 | 90 | 17 | 18 | Trace |
| C2H5OH | 13 | 86 | Trace | Trace | Trace |
| [1] |
Wilson, R. M.; Stockdill, J. L.; Wu, X.; Li, X.; Vadola, P. A.; Park, P. K.; Wang, P.; Danishefsky, S. J. Angew. Chem., Int. Ed. 2012, 51, 2834.
doi: 10.1002/anie.201106628 |
| [2] |
(a) Brown, M. F.; Mitton-Fry, M. J.; Arcari, J. T.; Barham, R.; Casavant, J.; Gerstenberger, B. S.; Han, S.; Hardink, J. R.; Harris, T. M.; Hoang, T.; Huband, M. D.; Lall, M. S.; Lemmon, M. M.; Li, C.; Lin, J.; McCurdy, S. P.; McElroy, E.; McPherson, C.; Marr, E. S.; Mueller, J. P.; Mullins, L.; Nikitenko, A. A.; Noe, M. C.; Penzien, J.; Plummer, M. S.; Schuff, B. P.; Shanmugasundaram, V.; Starr, J. T.; Sun, J.; Tomaras, A.; Young, J. A.; Zaniewski, R. P. J. Med. Chem. 2013, 56, 5541.
doi: 10.1021/jm400560z |
|
(b) Sharma, G. V. M.; Manohar, V.; Dutta, S. K.; Sridhar, B.; Ramesh, V.; Srinivas, R.; Kunwar, A. C. J. Org. Chem. 2010, 75, 1087.
doi: 10.1021/jo901923q |
|
|
(c) Yamawaki, K.; Nomura, T.; Yasukata, T.; Tanimoto, N.; Uotani, K.; Miwa, H.; Yamano, Y.; Takeda, K.; Nishitani, Y. Bioorg. Med. Chem. 2008, 16, 1632.
doi: 10.1016/j.bmc.2007.11.028 |
|
| [3] |
Ishii, T.; Kakeno, Y.; Nagao, K.; Ohmiya, H. J. Am. Chem. Soc. 2019, 141, 3854.
doi: 10.1021/jacs.9b00880 |
| [4] |
(a) Allen, L. J.; Cabrera, P. J.; Lee, M.; Sanford, M. S. J. Am. Chem. Soc. 2014, 136, 5607.
doi: 10.1021/ja501906x pmid: 28514176 |
|
(b) Qin, T.; Malins, L. R.; Edwards, J. T.; Merchant, R. R.; Novak, A. J.; Zhong, J. Z.; Mills, R. B.; Yan, M.; Yuan, C.; Eastgate, M. D.; Baran, P. S. Angew. Chem., Int. Ed. 2017, 56, 260.
doi: 10.1002/anie.201609662 pmid: 28514176 |
|
|
(c) Candish, L.; Teders, M.; Glorius, F. J. Am. Chem. Soc. 2017, 139, 7440.
doi: 10.1021/jacs.7b03127 pmid: 28514176 |
|
|
(d) Montesinos-Magraner, M.; Costantini, M.; Ramirez-Contreras, R.; Muratore, M. E.; Johansson, M. J.; Mendoza, A. Angew. Chem., Int. Ed. 2019, 58, 5930.
doi: 10.1002/anie.v58.18 pmid: 28514176 |
|
|
(e) Shu, C.; Noble, A.; Aggarwal, V. K. Nature 2020, 586, 714.
doi: 10.1038/s41586-020-2831-6 pmid: 28514176 |
|
| [5] |
(a) Xia, X. F.; Zhu, S. L.; Gu, Z.; Wang, H.; Li, W.; Liu, X.; Liang, Y. M. J. Org. Chem. 2015, 80, 5572.
doi: 10.1021/acs.joc.5b00460 |
|
(b) Xia, X.-F.; Zhu, S.-L.; Zhang, D. Tetrahedron 2015, 71, 8517.
doi: 10.1016/j.tet.2015.09.040 |
|
|
(c) Tang, S. Q.; Wang, A. P.; Schmitt, M.; Bihel, F. Tetrahedron Lett. 2018, 59, 1465.
doi: 10.1016/j.tetlet.2018.03.009 |
|
|
(d) Krylov, I. B.; Paveliev, S. A.; Matveeva, O. K.; Terent'ev, A. O. Tetrahedron 2019, 75, 2529.
doi: 10.1016/j.tet.2019.03.030 |
|
| [6] |
(a) Tan, B.; Toda, N.; Barbas, C. F. Angew. Chem., Int. Ed. 2012, 51, 12538.
doi: 10.1002/anie.201205921 pmid: 31886660 |
|
(b) Dinda, M.; Bose, C.; Ghosh, T.; Maity, S. RSC Adv. 2015, 2015, 44928.
pmid: 31886660 |
|
|
(c) Siddaraju, Y.; Prabhu, K. R. Org. Biomol. Chem. 2015, 13, 11651.
doi: 10.1039/c5ob01929j pmid: 31886660 |
|
|
(d) Lv, Y.; Sun, K.; Pu, W.; Mao, S.; Li, G.; Niu, J.; Chen, Q.; Wang, T. RSC Adv. 2016, 2016, 93486.
pmid: 31886660 |
|
|
(e) Su, W.; Jin, C.; Guo, Z.; Jiang, X.; Zhou, J.; Sun, B. Synlett 2017, 28, 1321.
doi: 10.1055/s-0036-1588760 pmid: 31886660 |
|
|
(f) Xu, X.; Li, P.; Huang, Y.; Tong, C.; Yan, Y.; Xie, Y. Tetrahedron Lett. 2017, 58, 1742.
doi: 10.1016/j.tetlet.2017.03.064 pmid: 31886660 |
|
|
(g) Xu, X.; Sun, J.; Lin, Y.; Cheng, J.; Li, P.; Jiang, X.; Bai, R.; Xie, Y. Eur. J. Org. Chem. 2017, 2017, 7160.
doi: 10.1002/ejoc.201701411 pmid: 31886660 |
|
|
(h) Feizpour, F.; Jafarpour, M.; Rezaeifard, A. New J. Chem. 2018, 42, 807.
doi: 10.1039/C7NJ03651E pmid: 31886660 |
|
|
(i) Joo, S.-R.; Kim, J.-S.; Park, S.-Y.; Kim, S.-H. Bull. Korean Chem. Soc. 2018, 39, 829.
doi: 10.1002/bkcs.2018.39.issue-6 pmid: 31886660 |
|
|
(j) Sun, B.; Wang, Y.; Li, D.; Jin, C.; Su, W. Org. Biomol. Chem. 2018, 16, 2902.
doi: 10.1039/C8OB00176F pmid: 31886660 |
|
|
(k) Krylov, I. B.; Lopat'eva, E. R.; Budnikov, A. S.; Nikishin, G. I.; Terent'ev, A. O. J. Org. Chem. 2020, 85, 1935.
doi: 10.1021/acs.joc.9b02656 pmid: 31886660 |
|
|
(l) Chen, R.; Liu, B.; Li, W.; Wang, K.-K.; Miao, C.; Li, Z.; Lv, Y.; Liu, L. RSC Adv. 2021, 11, 8051.
doi: 10.1039/D1RA00375E pmid: 31886660 |
|
| [7] |
(a) Singha, K.; Ghosh, S. C.; Panda, A. B. Chem.-Asian J. 2019, 14, 3205.
doi: 10.1002/asia.v14.18 |
|
(b) Singha, K.; Ghosh, S. C.; Panda, A. B. Eur. J. Org. Chem. 2021, 2021, 657.
doi: 10.1002/ejoc.v2021.4 |
|
| [8] |
Wu, F. F.; Han, X. Z.; Li, X. J.; Shen, X. B.; Wang, C.; Tian, Z. M.; Cheng, B.; Zhang, J. B.; Sheng, L. Q.; Zhai, H. B. Commun. Chem. 2021, 4, 46.
doi: 10.1038/s42004-021-00480-8 |
| [9] |
Tamura, Y.; Yakura, T.; Haruta, J.; Kita, Y. J. Org. Chem. 1987, 52, 3927.
doi: 10.1021/jo00226a041 |
| [10] |
Kita, Y.; Tohma, H.; Hatanaka, K.; Takada, T.; Fujita, S.; Mitoh, S.; Sakurai, H.; Oka, S. J. Am. Chem. Soc. 1994, 116, 3684.
doi: 10.1021/ja00088a003 |
| [11] |
Qian, P.-C.; Liu, Y.; Song, R.-J.; Hu, M.; Yang, X.-H.; Xiang, J.-N.; Li, J.-H. Eur. J. Org. Chem. 2015, 2015, 1680.
doi: 10.1002/ejoc.v2015.8 |
| [12] |
Bag, R.; Sar, D.; Punniyamurthy, T. Org. Lett. 2015, 17, 2010.
doi: 10.1021/acs.orglett.5b00770 |
| [13] |
Andia, A. A.; Miner, M. R.; Woerpel, K. A. Org. Lett. 2015, 17, 2704.
doi: 10.1021/acs.orglett.5b01120 |
| [14] |
Huihui, K. M.; Caputo, J. A.; Melchor, Z.; Olivares, A. M. J. Am. Chem. Soc. 2016, 138, 5016.
doi: 10.1021/jacs.6b01533 |
| [15] |
Chan, C.-M.; Xing, Q.; Chow, Y.-C.; Hung, S.-F.; Yu, W.-Y. Org. Lett. 2019, 21, 8037.
doi: 10.1021/acs.orglett.9b03020 |
| [16] |
Zhao, W.; Wurz, R. P.; Peters, J. C.; Fu, G. C. J. Am. Chem. Soc. 2017, 139, 12153.
doi: 10.1021/jacs.7b07546 pmid: 28841018 |
| [17] |
Cheng, W. M.; Shang, R.; Zhao, B.; Xing, W. L.; Fu, Y. Org. Lett. 2017, 19, 4291.
doi: 10.1021/acs.orglett.7b01950 |
| [1] | Luyao Li, Zhongwen He, Zhenguo Zhang, Zhenhua Jia, Teck-Peng Loh. Application of Triaryl Carbenium in Organic Synthesis [J]. Chinese Journal of Organic Chemistry, 2024, 44(2): 421-437. |
| [2] | Jie Liu, Feng Han, Shuangyan Li, Tianyu Chen, Jianhui Chen, Qing Xu. Transition Metal-Free Selective Aerobic Olefination of Methyl N-Heteroarenes with Alcohols [J]. Chinese Journal of Organic Chemistry, 2024, 44(2): 573-583. |
| [3] | Jianghu Dong, Liangming Xuan, Chi Wang, Chenxi Zhao, Haifeng Wang, Qiongjiao Yan, Wei Wang, Fen'er Chen. Recent Advances in Visible-Light-Induced C(3)—H Functionalization of Quinoxalinones under Transition-Metal-Free or Photocatalyst-Free [J]. Chinese Journal of Organic Chemistry, 2024, 44(1): 111-136. |
| [4] | Qianfan Zhao, Yongzheng Chen, Shiming Zhang. Application and Mechanism Study of Carbon-Based Metal-Free Catalysts in Organic Synthesis [J]. Chinese Journal of Organic Chemistry, 2024, 44(1): 137-147. |
| [5] | Yijun Shi, Xinyue Sun, Han Cao, Fusheng Bie, Jie Ma, Zhe Liu, Xingshun Cong. Thioesterification of Esters with Primary Aliphatic Thiols at Room Temperature [J]. Chinese Journal of Organic Chemistry, 2023, 43(7): 2499-2505. |
| [6] | Zhongrong Xu, Jieping Wan, Yunyun Liu. Transition Metal-Free C—H Thiocyanation and Selenocyanation Based on Thermochemical, Photocatalytic and Electrochemical Process [J]. Chinese Journal of Organic Chemistry, 2023, 43(7): 2425-2446. |
| [7] | Jiao Qin, Jie Chen, Yan Su. Synthesis of 2,2,6,6-Tetramethylpiperidin-1-yl-2-(2-cyanophenyl)-acetate by Transition Metal-Free Radical Cleavage Reaction from α-Bromoindanone [J]. Chinese Journal of Organic Chemistry, 2023, 43(6): 2171-2177. |
| [8] | Rui Wang, Lang Gao, Cen Zhou, Xiao Zhang. Haloperfluoroalkylation of Unactivated Terminal Alkenes over Phenylphenothiazine-Based Porous Organic Polymers [J]. Chinese Journal of Organic Chemistry, 2023, 43(3): 1136-1145. |
| [9] | Menghan Shen, Laiqiang Li, Quan Zhou, Jiehui Wang, Lei Wang. Visible-Light-Induced Regio-selective Oxidative Coupling of Quinoxalinones with Pyrrole Derivatives [J]. Chinese Journal of Organic Chemistry, 2023, 43(2): 697-704. |
| [10] | Biao Ma, Miaomiao Zhang, Zhanyu Li, Jinsong Peng, Chunxia Chen. Recent Advance of Transition Metal-Free Catalyzed Suzuki-Type Cross Coupling Reaction [J]. Chinese Journal of Organic Chemistry, 2023, 43(2): 455-470. |
| [11] | Yu Zhao, Yurong Duan, Shihui Shi, Yubin Bai, Liangzhu Huang, Xiaojun Yang, Yantu Zhang, Bin Feng, Jianbo Zhang, Qiuyu Zhang. Recent Advances of Hypervalent Iodine(III) Reagents upon Visible Light Irradiation [J]. Chinese Journal of Organic Chemistry, 2023, 43(12): 4106-4140. |
| [12] | Jing Sun, Mengmeng Zhang, Xiaolong Guo, Qi Wang, Luyao Wang. Synthesis of Diaryl Selenium Compounds without Transition-Metal Catalyst [J]. Chinese Journal of Organic Chemistry, 2023, 43(12): 4251-4260. |
| [13] | Duoduo Xiao, Jiantao Zhang, Peng Zhou, Weibing Liu. Metal-Free α-C(sp3)—H Methylenation of Aryl Ketones to Form γ-Keto Sulfoxides with Dimethyl Sulfoxide [J]. Chinese Journal of Organic Chemistry, 2023, 43(11): 3900-3906. |
| [14] | Qiyang Li, Haiyan Zhang, Wenbo Liu. Research Progress in Transition-Metal-Free C—Si Bond Formation [J]. Chinese Journal of Organic Chemistry, 2023, 43(10): 3470-3490. |
| [15] | Tianyu Chen, Feng Han, Shuangyan Li, Jianping Liu, Jianhui Chen, Qing Xu. Transition Metal-Free Selective Aerobic C-Alkylation of Methyl N-Heteroarenes with Alcohols [J]. Chinese Journal of Organic Chemistry, 2022, 42(9): 2914-2924. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||