Chinese Journal of Organic Chemistry ›› 2022, Vol. 42 ›› Issue (2): 607-618.DOI: 10.6023/cjoc202108009 Previous Articles Next Articles
ARTICLES
王伟a, 武复冉a, 马一丹a, 徐丹b,c,*( ), 徐功a,c,*(
), 徐功a,c,*( )
)
收稿日期:2021-08-07
修回日期:2021-09-18
发布日期:2022-02-24
通讯作者:
徐丹, 徐功
基金资助:
Wei Wanga, Furan Wua, Yidan Maa, Dan Xub,c( ), Gong Xua,c(
), Gong Xua,c( )
)
Received:2021-08-07
Revised:2021-09-18
Published:2022-02-24
Contact:
Dan Xu, Gong Xu
Supported by:Share
Wei Wang, Furan Wu, Yidan Ma, Dan Xu, Gong Xu. Study on Synthesis and Antifungal Activity of Novel Benzamides Containing Substituted Pyrazole Unit[J]. Chinese Journal of Organic Chemistry, 2022, 42(2): 607-618.
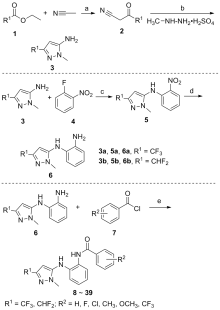

| 化合物 | R1 | R2 | 油菜菌核病菌 | 苹果腐烂病菌 | 烟草赤星病菌 | 玉米弯孢病菌 | 葡萄灰霉病菌 |
|---|---|---|---|---|---|---|---|
| 8 | CF3 | H | 52.08±1.71 | 50.72±3.17 | 56.57±2.11 | 42.25±1.03 | 38.13±7.11 |
| 9 | CF3 | 2-F | 81.22±3.54 | 64.31±6.88 | 73.68±0.86 | 45.42±1.42 | 58.52±2.44 |
| 10 | CF3 | 3-F | 71.73±1.99 | 62.84±1.41 | 59.21±0.41 | 41.27±1.17 | 52.86±3.33 |
| 11 | CF3 | 4-F | 67.96±5.25 | 60.68±4.58 | 65.45±1.04 | 41.28±2.74 | 47.90±4.21 |
| 12 | CF3 | 2-Cl | 80.70±1.16 | 68.91 ±4.56 | 61.52±0.93 | 38.14±3.43 | 54.97±5.46 |
| 13 | CF3 | 3-Cl | 87.99±1.96 | 82.38±1.30 | 64.47±0.66 | 47.21±0.53 | 68.82±3.08 |
| 14 | CF3 | 4-Cl | 89.72±2.72 | 86.72±0.61 | 66.25±0.73 | 49.60±1.20 | 55.60±2.15 |
| 15 | CF3 | 2-CF3 | 81.86±1.14 | 70.58±1.24 | 59.54±0.13 | 46.44±4.25 | 66.72±3.98 |
| 16 | CF3 | 3-CF3 | 35.08±4.33 | 31.68±5.21 | 20.39±1.38 | 13.35±2.49 | 37.88±2.83 |
| 17 | CF3 | 4-CF3 | 29.35±2.67 | 25.63±6.12 | 17.76±0.57 | 25.88±1.08 | 38.67±6.48 |
| 18 | CF3 | 2-CH3 | 61.85±3.47 | 54.59±5.01 | 48.03±0.28 | 36.23±4.24 | 48.50±1.02 |
| 19 | CF3 | 3-CH3 | 35.24±4.23 | 28.28±3.35 | 21.70±0.91 | 23.53±3.60 | 22.73±6.60 |
| 20 | CF3 | 4-CH3 | 82.80±1.40 | 89.95±0.63 | 58.22±1.45 | 50.38±1.98 | 55.41±2.72 |
| 21 | CF3 | 2-OCH3 | 65.30±2.03 | 54.53±4.30 | 51.98±0.75 | 24.39±1.32 | 36.47±3.42 |
| 22 | CF3 | 3-OCH3 | 64.64±3.29 | 60.27±5.18 | 54.28±0.59 | 39.25±2.33 | 33.06±1.46 |
| 23 | CF3 | 4-OCH3 | 75.43±1.04 | 79.72±1.55 | 55.19±1.53 | 47.05±3.34 | 50.42±3.87 |
| 24 | CHF2 | H | 62.64±3.40 | 32.89±5.39 | 35.85±0.75 | 47.75±3.03 | 33.47±4.14 |
| 25 | CHF2 | 2-F | 41.09±2.11 | 40.10±7.63 | 48.04±1.57 | 37.02±3.65 | 33.66±2.53 |
| 26 | CHF2 | 3-F | 60.73±7.33 | 36.71±6.59 | 39.15±1.27 | 25.37±1.05 | 51.45±8.28 |
| 27 | CHF2 | 4-F | 44.79±0.80 | 44.55±3.40 | 38.82±1.45 | 24.77±0.65 | 48.46±3.99 |
| 28 | CHF2 | 2-Cl | 55.77±1.94 | 56.96±4.78 | 50.98±0.48 | 35.40±1.87 | 72.36±3.68 |
| 29 | CHF2 | 3-Cl | 84.94±0.83 | 72.29±2.17 | 58.22±0.41 | 45.61±0.45 | 70.42±6.84 |
| 30 | CHF2 | 4-Cl | 83.42±0.36 | 73.18±2.35 | 52.31±1.06 | 47.56±0.67 | 51.88±4.87 |
| 31 | CHF2 | 2-CF3 | 49.10±0.68 | 46.60±5.94 | 41.11±0.52 | 32.65±0.33 | 32.28±6.58 |
| 32 | CHF2 | 3-CF3 | 80.02±1.48 | 66.90±3.16 | 56.58±0.14 | 33.15±3.38 | 73.25±3.92 |
| 33 | CHF2 | 4-CF3 | 30.49±4.19 | 31.32±5.49 | 25.00±2.40 | 14.43±1.23 | 63.35±7.24 |
| 34 | CHF2 | 2-CH3 | 43.27±5.48 | 42.28±2.61 | 41.77±1.17 | 32.05±0.54 | 54.95±7.68 |
| 35 | CHF2 | 3-CH3 | 44.09±2.12 | 59.53±2.66 | 46.71±0.91 | 27.71±1.23 | 25.06±0.81 |
| 36 | CHF2 | 4-CH3 | 70.16±1.97 | 55.18±2.34 | 44.74±0.60 | 43.86±2.85 | 28.40±4.13 |
| 37 | CHF2 | 2-OCH3 | 63.44±1.42 | 58.43±1.58 | 56.25±0.73 | 37.29±2.16 | 36.51±3.80 |
| 38 | CHF2 | 3-OCH3 | 63.48±1.50 | 38.35±3.45 | 42.76±0.60 | 44.24±3.42 | 24.84±6.94 |
| 39 | CHF2 | 4-OCH3 | 46.79±4.29 | 44.15±3.47 | 45.73±1.55 | 36.98±3.94 | 25.32±4.76 |
| 5IIcc | CHF2 | 3-CH3 | 100 | 95.00±0.41 | 67.62±2.24 | 52.42±1.34 | 89.33±1.92 |
| 氟唑菌酰胺b | 100 | 81.30±2.70 | 95.07±0.65 | 100 | 63.16±1.24 |
| 化合物 | R1 | R2 | 油菜菌核病菌 | 苹果腐烂病菌 | 烟草赤星病菌 | 玉米弯孢病菌 | 葡萄灰霉病菌 |
|---|---|---|---|---|---|---|---|
| 8 | CF3 | H | 52.08±1.71 | 50.72±3.17 | 56.57±2.11 | 42.25±1.03 | 38.13±7.11 |
| 9 | CF3 | 2-F | 81.22±3.54 | 64.31±6.88 | 73.68±0.86 | 45.42±1.42 | 58.52±2.44 |
| 10 | CF3 | 3-F | 71.73±1.99 | 62.84±1.41 | 59.21±0.41 | 41.27±1.17 | 52.86±3.33 |
| 11 | CF3 | 4-F | 67.96±5.25 | 60.68±4.58 | 65.45±1.04 | 41.28±2.74 | 47.90±4.21 |
| 12 | CF3 | 2-Cl | 80.70±1.16 | 68.91 ±4.56 | 61.52±0.93 | 38.14±3.43 | 54.97±5.46 |
| 13 | CF3 | 3-Cl | 87.99±1.96 | 82.38±1.30 | 64.47±0.66 | 47.21±0.53 | 68.82±3.08 |
| 14 | CF3 | 4-Cl | 89.72±2.72 | 86.72±0.61 | 66.25±0.73 | 49.60±1.20 | 55.60±2.15 |
| 15 | CF3 | 2-CF3 | 81.86±1.14 | 70.58±1.24 | 59.54±0.13 | 46.44±4.25 | 66.72±3.98 |
| 16 | CF3 | 3-CF3 | 35.08±4.33 | 31.68±5.21 | 20.39±1.38 | 13.35±2.49 | 37.88±2.83 |
| 17 | CF3 | 4-CF3 | 29.35±2.67 | 25.63±6.12 | 17.76±0.57 | 25.88±1.08 | 38.67±6.48 |
| 18 | CF3 | 2-CH3 | 61.85±3.47 | 54.59±5.01 | 48.03±0.28 | 36.23±4.24 | 48.50±1.02 |
| 19 | CF3 | 3-CH3 | 35.24±4.23 | 28.28±3.35 | 21.70±0.91 | 23.53±3.60 | 22.73±6.60 |
| 20 | CF3 | 4-CH3 | 82.80±1.40 | 89.95±0.63 | 58.22±1.45 | 50.38±1.98 | 55.41±2.72 |
| 21 | CF3 | 2-OCH3 | 65.30±2.03 | 54.53±4.30 | 51.98±0.75 | 24.39±1.32 | 36.47±3.42 |
| 22 | CF3 | 3-OCH3 | 64.64±3.29 | 60.27±5.18 | 54.28±0.59 | 39.25±2.33 | 33.06±1.46 |
| 23 | CF3 | 4-OCH3 | 75.43±1.04 | 79.72±1.55 | 55.19±1.53 | 47.05±3.34 | 50.42±3.87 |
| 24 | CHF2 | H | 62.64±3.40 | 32.89±5.39 | 35.85±0.75 | 47.75±3.03 | 33.47±4.14 |
| 25 | CHF2 | 2-F | 41.09±2.11 | 40.10±7.63 | 48.04±1.57 | 37.02±3.65 | 33.66±2.53 |
| 26 | CHF2 | 3-F | 60.73±7.33 | 36.71±6.59 | 39.15±1.27 | 25.37±1.05 | 51.45±8.28 |
| 27 | CHF2 | 4-F | 44.79±0.80 | 44.55±3.40 | 38.82±1.45 | 24.77±0.65 | 48.46±3.99 |
| 28 | CHF2 | 2-Cl | 55.77±1.94 | 56.96±4.78 | 50.98±0.48 | 35.40±1.87 | 72.36±3.68 |
| 29 | CHF2 | 3-Cl | 84.94±0.83 | 72.29±2.17 | 58.22±0.41 | 45.61±0.45 | 70.42±6.84 |
| 30 | CHF2 | 4-Cl | 83.42±0.36 | 73.18±2.35 | 52.31±1.06 | 47.56±0.67 | 51.88±4.87 |
| 31 | CHF2 | 2-CF3 | 49.10±0.68 | 46.60±5.94 | 41.11±0.52 | 32.65±0.33 | 32.28±6.58 |
| 32 | CHF2 | 3-CF3 | 80.02±1.48 | 66.90±3.16 | 56.58±0.14 | 33.15±3.38 | 73.25±3.92 |
| 33 | CHF2 | 4-CF3 | 30.49±4.19 | 31.32±5.49 | 25.00±2.40 | 14.43±1.23 | 63.35±7.24 |
| 34 | CHF2 | 2-CH3 | 43.27±5.48 | 42.28±2.61 | 41.77±1.17 | 32.05±0.54 | 54.95±7.68 |
| 35 | CHF2 | 3-CH3 | 44.09±2.12 | 59.53±2.66 | 46.71±0.91 | 27.71±1.23 | 25.06±0.81 |
| 36 | CHF2 | 4-CH3 | 70.16±1.97 | 55.18±2.34 | 44.74±0.60 | 43.86±2.85 | 28.40±4.13 |
| 37 | CHF2 | 2-OCH3 | 63.44±1.42 | 58.43±1.58 | 56.25±0.73 | 37.29±2.16 | 36.51±3.80 |
| 38 | CHF2 | 3-OCH3 | 63.48±1.50 | 38.35±3.45 | 42.76±0.60 | 44.24±3.42 | 24.84±6.94 |
| 39 | CHF2 | 4-OCH3 | 46.79±4.29 | 44.15±3.47 | 45.73±1.55 | 36.98±3.94 | 25.32±4.76 |
| 5IIcc | CHF2 | 3-CH3 | 100 | 95.00±0.41 | 67.62±2.24 | 52.42±1.34 | 89.33±1.92 |
| 氟唑菌酰胺b | 100 | 81.30±2.70 | 95.07±0.65 | 100 | 63.16±1.24 |
| 真菌 | 化合物 | R1 | R2 | EC50/(mg•L–1) | 95%置信区间 | 毒力回归方程 | R2 |
|---|---|---|---|---|---|---|---|
| 油菜菌核病菌 | 9 | CF3 | 2-F | 18.90 | 11.96~37.56 | y=–1.684+1.319x | 0.931 |
| 10 | CF3 | 3-F | 21.58 | 16.37~30.82 | y=–1.289+0.966x | 0.932 | |
| 11 | CF3 | 4-F | 30.11 | 23.06~42.76 | y=–1.687+1.141x | 0.948 | |
| 12 | CF3 | 2-Cl | 20.07 | 12.49~42.19 | y=–1.772+1.360x | 0.929 | |
| 13 | CF3 | 3-Cl | 15.09 | 9.36~28.97 | y=–1.718+1.457x | 0.925 | |
| 14 | CF3 | 4-Cl | 11.21 | 7.32~18.12 | y=–1.613+1.537x | 0.941 | |
| 15 | CF3 | 2-CF3 | 14.74 | 9.43~26.83 | y=–1.360+1.164x | 0.927 | |
| 20 | CF3 | 4-CH3 | 15.99 | 9.72~32.97 | y=–1.492+1.240x | 0.920 | |
| 23 | CF3 | 4-OCH3 | 21.98 | 13.64~47.94 | y=–1.595+1.188x | 0.932 | |
| 29 | CHF2 | 3-Cl | 17.96 | 11.24~35.80 | y=–1.859+1.482x | 0.931 | |
| 30 | CHF2 | 4-Cl | 18.73 | 11.85~37.00 | y=–1.864+1.464x | 0.935 | |
| 32 | CHF2 | 3-CF3 | 19.49 | 11.86~43.08 | y=–1.639+1.271x | 0.921 | |
| 36 | CHF2 | 4-CH3 | 23.76 | 18.25~33.32 | y=–1.451+1.055x | 0.957 | |
| 氟唑菌酰胺a | 0.55 | 0.20~0.98 | y=0.253+0.974x | 0.930 | |||
| 苹果腐烂病菌 | 9 | CF3 | 2-F | 28.46 | 19.88~47.86 | y=–1.150+0.91x | 0.926 |
| 10 | CF3 | 3-F | 27.26 | 18.52~48.47 | y=–1.025+0.714x | 0.915 | |
| 11 | CF3 | 4-F | 28.84 | 19.18~53.99 | y=–0.999+0.684x | 0.958 | |
| 12 | CF3 | 2-Cl | 20.86 | 14.98~32.71 | y=–1.028+0.779x | 0.918 | |
| 13 | CF3 | 3-Cl | 11.17 | 6.91~19.58 | y=–1.249+1.191x | 0.918 | |
| 14 | CF3 | 4-Cl | 11.67 | 7.51~19.69 | y=–1.138+1.067x | 0.933 | |
| 15 | CF3 | 2-CF3 | 19.23 | 14.12~28.78 | y=–1.059+0.829x | 0.928 | |
| 20 | CF3 | 4-CH3 | 16.14 | 9.92~32.04 | y=–1.957+1.620x | 0.922 | |
| 23 | CHF2 | 4-OCH3 | 16.23 | 10.87~28.38 | y=–1.423+1.175x | 0.939 | |
| 29 | CHF2 | 3-Cl | 16.01 | 11.98~22.91 | y=–1.019+0.846x | 0.940 | |
| 30 | CHF2 | 4-Cl | 18.86 | 14.56~25.99 | y=–1.277+1.001x | 0.946 | |
| 32 | CHF2 | 3-CF3 | 20.83 | 16.63~27.36 | y=–1.599+1.212x | 0.961 | |
| 36 | CHF2 | 4-CH3 | 46.72 | 31.92~82.28 | y=–1.528+0.916x | 0.974 | |
| 氟唑菌酰胺a | 16.49 | 11.81~27.49 | y=–1.112+0.914x | 0.984 | |||
| 烟草赤星病菌 | 9 | CF3 | 2-F | 17.19 | 4.89~28.20 | y=–1.627+1.317x | 0.990 |
| 10 | CF3 | 3-F | 39.89 | 26.67~73.75 | y=–1.274+0.796x | 0.944 | |
| 11 | CF3 | 4-F | 26.60 | 15.58~71.12 | y=–1.333+0.936x | 0.906 | |
| 12 | CF3 | 2-Cl | 31.06 | 21.76~51.93 | y=–1.234+0.827x | 0.934 | |
| 13 | CF3 | 3-Cl | 25.89 | 19.82~36.60 | y=–1.514+1.071x | 0.979 | |
| 14 | CF3 | 4-Cl | 16.70 | 13.64~20.91 | y=–1.162+0.858x | 0.959 | |
| 15 | CF3 | 2-CF3 | 32.58 | 25.23~70.83 | y=–1.142+0.755x | 0.963 | |
| 20 | CF3 | 4-CH3 | 34.97 | 3.45~62.57 | y=–1.568+1.016x | 0.946 | |
| 23 | CF3 | 4-OCH3 | 33.02 | 21.90~62.36 | y=–1.088+0.716x | 0.938 | |
| 29 | CHF2 | 3-Cl | 37.16 | 24.82~69.89 | y=–1.383+0.881x | 0.948 | |
| 30 | CHF2 | 4-Cl | 50.71 | 31.21~112.50 | y=–0.984+0.577x | 0.993 | |
| 32 | CHF2 | 3-CF3 | 46.83 | 30.65~90.41 | y=–1.344+0.804x | 0.922 | |
| 氟唑菌酰胺a | 0.68 | 0.23~1.27 | y=0.125+0.773x | 0.941 |
| 真菌 | 化合物 | R1 | R2 | EC50/(mg•L–1) | 95%置信区间 | 毒力回归方程 | R2 |
|---|---|---|---|---|---|---|---|
| 油菜菌核病菌 | 9 | CF3 | 2-F | 18.90 | 11.96~37.56 | y=–1.684+1.319x | 0.931 |
| 10 | CF3 | 3-F | 21.58 | 16.37~30.82 | y=–1.289+0.966x | 0.932 | |
| 11 | CF3 | 4-F | 30.11 | 23.06~42.76 | y=–1.687+1.141x | 0.948 | |
| 12 | CF3 | 2-Cl | 20.07 | 12.49~42.19 | y=–1.772+1.360x | 0.929 | |
| 13 | CF3 | 3-Cl | 15.09 | 9.36~28.97 | y=–1.718+1.457x | 0.925 | |
| 14 | CF3 | 4-Cl | 11.21 | 7.32~18.12 | y=–1.613+1.537x | 0.941 | |
| 15 | CF3 | 2-CF3 | 14.74 | 9.43~26.83 | y=–1.360+1.164x | 0.927 | |
| 20 | CF3 | 4-CH3 | 15.99 | 9.72~32.97 | y=–1.492+1.240x | 0.920 | |
| 23 | CF3 | 4-OCH3 | 21.98 | 13.64~47.94 | y=–1.595+1.188x | 0.932 | |
| 29 | CHF2 | 3-Cl | 17.96 | 11.24~35.80 | y=–1.859+1.482x | 0.931 | |
| 30 | CHF2 | 4-Cl | 18.73 | 11.85~37.00 | y=–1.864+1.464x | 0.935 | |
| 32 | CHF2 | 3-CF3 | 19.49 | 11.86~43.08 | y=–1.639+1.271x | 0.921 | |
| 36 | CHF2 | 4-CH3 | 23.76 | 18.25~33.32 | y=–1.451+1.055x | 0.957 | |
| 氟唑菌酰胺a | 0.55 | 0.20~0.98 | y=0.253+0.974x | 0.930 | |||
| 苹果腐烂病菌 | 9 | CF3 | 2-F | 28.46 | 19.88~47.86 | y=–1.150+0.91x | 0.926 |
| 10 | CF3 | 3-F | 27.26 | 18.52~48.47 | y=–1.025+0.714x | 0.915 | |
| 11 | CF3 | 4-F | 28.84 | 19.18~53.99 | y=–0.999+0.684x | 0.958 | |
| 12 | CF3 | 2-Cl | 20.86 | 14.98~32.71 | y=–1.028+0.779x | 0.918 | |
| 13 | CF3 | 3-Cl | 11.17 | 6.91~19.58 | y=–1.249+1.191x | 0.918 | |
| 14 | CF3 | 4-Cl | 11.67 | 7.51~19.69 | y=–1.138+1.067x | 0.933 | |
| 15 | CF3 | 2-CF3 | 19.23 | 14.12~28.78 | y=–1.059+0.829x | 0.928 | |
| 20 | CF3 | 4-CH3 | 16.14 | 9.92~32.04 | y=–1.957+1.620x | 0.922 | |
| 23 | CHF2 | 4-OCH3 | 16.23 | 10.87~28.38 | y=–1.423+1.175x | 0.939 | |
| 29 | CHF2 | 3-Cl | 16.01 | 11.98~22.91 | y=–1.019+0.846x | 0.940 | |
| 30 | CHF2 | 4-Cl | 18.86 | 14.56~25.99 | y=–1.277+1.001x | 0.946 | |
| 32 | CHF2 | 3-CF3 | 20.83 | 16.63~27.36 | y=–1.599+1.212x | 0.961 | |
| 36 | CHF2 | 4-CH3 | 46.72 | 31.92~82.28 | y=–1.528+0.916x | 0.974 | |
| 氟唑菌酰胺a | 16.49 | 11.81~27.49 | y=–1.112+0.914x | 0.984 | |||
| 烟草赤星病菌 | 9 | CF3 | 2-F | 17.19 | 4.89~28.20 | y=–1.627+1.317x | 0.990 |
| 10 | CF3 | 3-F | 39.89 | 26.67~73.75 | y=–1.274+0.796x | 0.944 | |
| 11 | CF3 | 4-F | 26.60 | 15.58~71.12 | y=–1.333+0.936x | 0.906 | |
| 12 | CF3 | 2-Cl | 31.06 | 21.76~51.93 | y=–1.234+0.827x | 0.934 | |
| 13 | CF3 | 3-Cl | 25.89 | 19.82~36.60 | y=–1.514+1.071x | 0.979 | |
| 14 | CF3 | 4-Cl | 16.70 | 13.64~20.91 | y=–1.162+0.858x | 0.959 | |
| 15 | CF3 | 2-CF3 | 32.58 | 25.23~70.83 | y=–1.142+0.755x | 0.963 | |
| 20 | CF3 | 4-CH3 | 34.97 | 3.45~62.57 | y=–1.568+1.016x | 0.946 | |
| 23 | CF3 | 4-OCH3 | 33.02 | 21.90~62.36 | y=–1.088+0.716x | 0.938 | |
| 29 | CHF2 | 3-Cl | 37.16 | 24.82~69.89 | y=–1.383+0.881x | 0.948 | |
| 30 | CHF2 | 4-Cl | 50.71 | 31.21~112.50 | y=–0.984+0.577x | 0.993 | |
| 32 | CHF2 | 3-CF3 | 46.83 | 30.65~90.41 | y=–1.344+0.804x | 0.922 | |
| 氟唑菌酰胺a | 0.68 | 0.23~1.27 | y=0.125+0.773x | 0.941 |
| [1] |
Liu, Z.; Li, Q. X.; Song, B. J. Agric. Food Chem. 2020, 68, 11039.
doi: 10.1021/acs.jafc.0c02376 |
| [2] |
Fisher, M. C.; Hawkins, N. J.; Sanglard, D.; Gurr, S. J. Science 2018, 360, 739.
doi: 10.1126/science.aap7999 |
| [3] |
Wang, W.; Zhang, S.; Wang, J. H.; Wu, F. R.; Wang, T.; Xu, G. J. Agric. Food Chem. 2021, 69, 491.
doi: 10.1021/acs.jafc.0c06700 |
| [4] |
Patriarca, A. Curr. Opin. Food Sci. 2019, 29, 42.
doi: 10.1016/j.cofs.2019.08.002 |
| [5] |
Wang, W.; Cheng, X.; Cui, X.; Xia, D. G.; Wang, Z. Q.; Lü, X. H. Pest Manage. Sci. 2021, 77, 3529.
doi: 10.1002/ps.v77.7 |
| [6] |
Ishii, H.; Zhen, F.; Hu, M. J.; Li, X. P.; Schnabel, G. Pest Manage. Sci. 2016, 72, 1844.
doi: 10.1002/ps.4216 |
| [7] |
Li, H.; Gao, M. Q.; Chen, Y.; Wang, Y. X.; Yang, G. F. J. Agric. Food Chem. 2020, 68, 14001.
doi: 10.1021/acs.jafc.0c05646 |
| [8] |
Wei, G.; Huang, M.-W.; Wang, W.-J.; Wu, Y.; Mei, S.-F.; Zhou, L.-M.; Mei, L.-C.; Zhu, X.-L.; Yang, G.-F. J. Agric. Food Chem. 2021, 69, 3965.
doi: 10.1021/acs.jafc.0c07322 |
| [9] |
Xiong, L.; Li, H.; Jiang, L.-N.; Ge, J.-M.; Yang, W.-C.; Zhu, X. L.; Yang, G.-F. J. Agric. Food Chem. 2017, 65, 1021.
doi: 10.1021/acs.jafc.6b05134 |
| [10] |
Xiong, L.; Zhu, X.-L.; Gao, H.-W.; Fu, Y.; Hu, S.-Q.; Jiang, L.-N.; Yang, W.-C.; Yang, G.-F. J. Agric. Food Chem. 2016, 64, 4830.
doi: 10.1021/acs.jafc.6b00325 |
| [11] |
Xiong, L.; Shen, Y.-Q.; Jiang, L.-N.; Zhu, X.-L.; Yang, W.-C.; Huang, W.; Yang, G.-F. ACS Symp. Ser. 2015, 1204, 175.
|
| [12] |
Bardas, G. A.; Veloukas, T.; Koutita, O.; Karaoglanidis, G. S. Pest Manage. Sci. 2010, 66, 967.
doi: 10.1002/ps.v66:9 |
| [13] |
Xiong, L.; Zhu, X.-L.; Shen, Y.-Q.; Wishwa, W. K. W. M.; Li, K.; Yang, G.-F. Eur. J. Med. Chem. 2015, 95, 424.
doi: 10.1016/j.ejmech.2015.03.060 pmid: 25841198 |
| [14] |
Guo, X. F.; Zhao, B.; Fan, Z. J.; Yan, D. Y.; Zhang, N. L.; Wu, Q. F.; Yu, B.; Zhou, S.; Kalinina, T. A.; Belskaya, N. P. J. Agric. Food Chem. 2019, 67, 1647.
doi: 10.1021/acs.jafc.8b06935 |
| [15] |
Jones, D. Nat. Rev. Drug Discovery 2010, 9, 751.
doi: 10.1038/nrd3289 |
| [16] |
Wang, W.; Wang, J. H.; Wu, F. R.; Zhou, H.; Xu, D.; Xu, G. J. Agric. Food Chem. 2021, 69, 5746.
doi: 10.1021/acs.jafc.0c08094 |
| [17] |
Katte, T. A.; Reekie, T. A.; Jorgensen, W. T.; Kassiou, M. J. Org. Chem. 2016, 81, 4883.
doi: 10.1021/acs.joc.6b00710 |
| [18] |
Li, A.; Li, Z.; Zhao, Y.; Yao, T.; Zhao, J. Chin. J. Org. Chem. 2020, 40, 2836. (in Chinese)
doi: 10.6023/cjoc202004013 |
|
( 李安邦, 李中珊, 赵洋, 姚停停, 程敬丽, 赵金浩, 有机化学, 2020, 40, 2836.)
doi: 10.6023/cjoc202004013 |
|
| [19] |
Liu, H.; Xia, D. G.; Hu, R.; Wang, W.; Cheng, X.; Wang, A. L.; Zhang, Q.; Lü, X. H. Pestic. Biochem. Physiol. 2020, 163, 271.
doi: 10.1016/j.pestbp.2019.11.024 |
| [20] |
Lü, X. H.; Ren, Z. L.; Liu, P.; Li, B. X.; Li, Q. S.; Chu, M. J.; Cao, H. Q. Pest Manage. Sci. 2017, 73, 1585.
doi: 10.1002/ps.2017.73.issue-8 |
| [21] |
Zhu, X. L.; Xiong, L.; Li, H.; Song, X. Y.; Liu, J. J.; Yang, G. F. ChemMedChem 2014, 9, 1512.
doi: 10.1002/cmdc.v9.7 |
| [1] | Haibo Huo, Guixia Li, Shijun Wang, Chun Han, Baojun Shi, Jian Li. Novel γ-Carboline Derivatives as Antibacterial Agents: Synthesis and Antibacterial Evaluation [J]. Chinese Journal of Organic Chemistry, 2024, 44(1): 204-215. |
| [2] | Hu Ma, Danfeng Huang, Kehu Wang, Duoduo Tang, Yang Feng, Yuanyuan Reng, Junjiao Wang, Yulai Hu. Synthesis of 3-Trifluoromethylpyrazole Derivatives [J]. Chinese Journal of Organic Chemistry, 2023, 43(9): 3257-3267. |
| [3] | Feng Wang, Yu Chen, Hongyan Pei, Jing Zhang, Lixin Zhang. Design, Synthesis and Antifungal Activities of Novel 1,2,4-Oxadiazole Derivatives Containing Piperidine [J]. Chinese Journal of Organic Chemistry, 2023, 43(8): 2826-2836. |
| [4] | Rongchao Lei, Wenjie Lan, Mengzhu Li, Bin Fu. A Convenient Approach to Benzosultam-Linked Pyrazole Compounds [J]. Chinese Journal of Organic Chemistry, 2023, 43(7): 2553-2560. |
| [5] | Yang Sun, Yang Wang, Zichan Zhang, Ye Qian, Guicheng Luo, Beibei Zhou, Lisha Miao, Yudie Chen, Hong Dai, Baolin Xu, Zhengguang Wu. Synthesis and Bioactivities of Novel Pyrazole Oxime Derivatives Containing 1,3,4-Oxadiazole Group [J]. Chinese Journal of Organic Chemistry, 2023, 43(4): 1584-1590. |
| [6] | Zichan Zhang, Yang Sun, Sheng Hua, Baolin Xu, Min Zhang, Qin Zhao, Dandan Zheng, Yang Wang, Jianfeng Ju, Yujun Shi, Hong Dai. Synthesis and Insecticidal Activity of Novel Pyrazole Amides Containing an Isoxazole Moiety [J]. Chinese Journal of Organic Chemistry, 2023, 43(4): 1435-1443. |
| [7] | Xingzhou Liu, Mingjia Yu, Jianhua Liang. Research Progress on the Synthesis of Protoberberine Skeleton and Its Anti-inflammatory Activity [J]. Chinese Journal of Organic Chemistry, 2023, 43(4): 1325-1340. |
| [8] | Meng Li, Dongguo Xia, Yunxiao Wang, Xiang Cheng, Jiexiu Gong, Yao Chen, Xianhai Lü. Design, Synthesis and Antifungal Bioactivity Evaluation of Thiazole Benzoate Derivatives [J]. Chinese Journal of Organic Chemistry, 2023, 43(2): 686-696. |
| [9] | Rong Zhang, Xiang Gao, Lingling Chen, Fajun Nan. Discovery and Structure-Activity Relationship Studies of Thiazole- Oxazole Tandem Heterocyclic RNA Splicing Inhibitors [J]. Chinese Journal of Organic Chemistry, 2022, 42(9): 2925-2939. |
| [10] | Wei Chen, Simin Lei, Yuxin Lan, Haojian Xu, Pingbin Yu, Rui Zhang, Run Wu, Yang Chen. Design, Synthesis and Antifungal Activities of Novel Quinazolinone Derivatives [J]. Chinese Journal of Organic Chemistry, 2022, 42(7): 2164-2171. |
| [11] | Haojie Ma, Fengyuan Zhou, Jinlei Liu, Bo Han, Hua Yang, Yuqi Zhang, Jijiang Wang. Construction of Substituted N-Phenylpyrazoles via a Catalyst- Free and Additive-Free Intermolecular Cyclization Process [J]. Chinese Journal of Organic Chemistry, 2022, 42(6): 1843-1848. |
| [12] | Changkai Wang, Tengda Sun, Xuebo Zhang, Xinling Yang, Xingxing Lu, Huan Xu, Fasheng Shi, Li Zhang, Yun Ling. Design, Synthesis and Bioactivity of Novel Fluoropyrazole Hydrazides [J]. Chinese Journal of Organic Chemistry, 2022, 42(5): 1527-1536. |
| [13] | Siyu Zhu, Xinyu Huo, Qin Ma, Wei Chen, Jie Zhang, Liang Guo. Design, Synthesis, and Antitumor Activity of β-Carboline-Benzimidazole Hybrids [J]. Chinese Journal of Organic Chemistry, 2022, 42(4): 1129-1135. |
| [14] | Xiu Wang, Wengui Duan, Guishan Lin, Baoyu Li, Wenjing Zhang, Fuhou Lei. Synthesis, Antifungal Activity, Three-Dimensional Quantitative Structure-Activity Relationship and Molecular Docking Study of 4-Acyl-3-amino-1,2,4-triazole-thioether Derivatives Containing Natural Pinene Structure [J]. Chinese Journal of Organic Chemistry, 2022, 42(3): 871-883. |
| [15] | Yan Zeng, Lifei Nie, Chao Niu, Aytilla Mamatjan, Khurshed Bozorov, Jiangyu Zhao, Haji Akber Aisa. Synthesis and Biological Activities of Dihydrooxazolo[5,4-d]-pyrrolo[1,2-a]pyrimidinones [J]. Chinese Journal of Organic Chemistry, 2022, 42(2): 543-556. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||