Chinese Journal of Organic Chemistry ›› 2022, Vol. 42 ›› Issue (7): 2028-2044.DOI: 10.6023/cjoccjoc202203014 Previous Articles Next Articles
REVIEWS
收稿日期:2022-03-04
修回日期:2022-03-26
发布日期:2022-08-09
通讯作者:
贾丰成, 吴安心
基金资助:
Zexin Huang, Yuqiang Yina, Fengcheng Jia( ), Anxin Wua(
), Anxin Wua( )
)
Received:2022-03-04
Revised:2022-03-26
Published:2022-08-09
Contact:
Fengcheng Jia, Anxin Wu
Supported by:Share
Zexin Huang, Yuqiang Yin, Fengcheng Jia, Anxin Wu. Research Progress on C2—C3 Bond Cleavage of Indole and Its Derivatives[J]. Chinese Journal of Organic Chemistry, 2022, 42(7): 2028-2044.
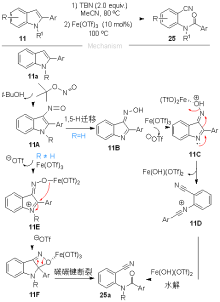

| [1] |
For selected papers, see: (a) Stempel, E.; Gaich, T. Acc. Chem. Res. 2016, 49, 2390.
doi: 10.1021/acs.accounts.6b00265 |
|
(b) Zi, W.; Zuo, Z.; Ma, D. Acc. Chem. Res. 2015, 48, 702.
doi: 10.1021/ar5004303 |
|
|
(c) Xu, Z.; Wang, Q.; Zhu, J. Chem. Soc. Rev. 2018, 47, 7882.
doi: 10.1039/C8CS00454D |
|
| [2] |
(a) Wang, W.; Zhang, M.; Yang, W.; Yang, X. Chin. J. Org. Chem. 2022, 42, 75. (in Chinese)
doi: 10.6023/cjoc202107012 |
|
( 王弯弯, 张明明, 杨文超, 杨小虎, 有机化学, 2022, 42, 75.)
doi: 10.6023/cjoc202107012 |
|
|
(b) Shi, Z.; Nie, K.; Liu, C.; Zhang, M.; Zhang, W. Chin. J. Org. Chem. 2020, 40, 327. (in Chinese)
doi: 10.6023/cjoc201907047 |
|
|
( 施展, 聂克睿, 刘畅, 张明智, 章维华, 有机化学, 2020, 40, 327.)
doi: 10.6023/cjoc201907047 |
|
| [3] |
For selected examples, see: (a) Robinson, B. Chem. Rev. 1963, 63, 373.
doi: 10.1021/cr60224a003 |
|
(b) Cacchi, S.; Fabrizi, G. Chem. Rev. 2005, 105, 2873.
doi: 10.1021/cr040639b |
|
|
(d) Neto, J. S. S.; Zeni, G. Org. Chem. Front. 2020, 7, 155.
doi: 10.1039/C9QO01315F |
|
| [4] |
(a) Luo, J.; Xu, X.; Zheng, J. Chin. J. Org. Chem. 2018, 38, 363. (in Chinese)
doi: 10.6023/cjoc201707031 |
|
( 骆钧飞, 徐星, 郑俊良, 有机化学, 2018, 38, 363.)
doi: 10.6023/cjoc201707031 |
|
|
(b) Wen, J.; Shi, Z. Acc. Chem. Res. 2021, 54, 1723.
doi: 10.1021/acs.accounts.0c00888 |
|
|
(d) Festa, A. A.; Voskressensky, L. G.; Van der Eycken, E. V. Chem. Soc. Rev. 2019, 48, 4401.
doi: 10.1039/C8CS00790J |
|
|
(c) Liu, S.; Zhao, F.; Chen, X.; Deng, G.; Huang, H. Adv. Synth. Catal. 2020, 362, 3795.
doi: 10.1002/adsc.202000285 |
|
|
(d) Huang, G.; Yin, B. Adv. Synth. Catal., 2018, 361, 405.
doi: 10.1002/adsc.201800789 |
|
| [5] |
Shi, Z.; Zhang, B.; Cui, Y.; Jiao, N. Angew. Chem. Int. Ed. 2010, 49, 4036.
doi: 10.1002/anie.201001237 |
| [6] |
Gangadhararao, G.; Uruvakilli, A.; Swamy, K. C. K. Org. Lett. 2014, 16, 6060.
doi: 10.1021/ol502783j pmid: 25402216 |
| [7] |
Bakthadoss, M.; Kumar, P. V.; Reddy, T. T.; Sharada, D. S. Org. Biomol. Chem. 2018, 16, 8160.
doi: 10.1039/c8ob01825a pmid: 30346008 |
| [8] |
Lian, X.-L.; Lei, H.; Quan, X.-J.; Ren, Z.-H.; Wang, Y.-Y.; Guan, Z.-H. Chem. Commun. 2013, 49, 8196.
doi: 10.1039/c3cc44215b |
| [9] |
Feng, Y.; Li, Y.; Cheng, G.; Wang, L.; Cui, X. J. Org. Chem. 2015, 80, 7099.
doi: 10.1021/acs.joc.5b00957 |
| [10] |
Yamashita, M.; Nishizono, Y.; Himekawa, S.; Iida, A. Tetrahedron 2016, 72, 4123.
doi: 10.1016/j.tet.2016.05.054 |
| [11] |
Liu, X. X.; Luo, X. L.; Wu, Z. Y.; Cui, X. F.; Zhou, X. Q.; He, Y. Q.; Huang, G. S. J. Org. Chem. 2017, 82, 2107.
doi: 10.1021/acs.joc.6b02893 |
| [12] |
Cao, W. B.; Chu, X. Q.; Zhou, Y.; Yin, L.; Xu, X. P.; Ji, S. J. Chem. Commun. 2017, 53, 6601.
doi: 10.1039/C7CC02815F |
| [13] |
Zhang, L. L.; Cao, W. B.; Xu, X. P.; Ji, S. J. Org. Chem. Front. 2019, 6, 1787.
doi: 10.1039/C9QO00379G |
| [14] |
Cao, W. B.; Liu, B. B.; Xu, X. P.; Ji, S. J. Org. Chem. Front. 2018, 5, 1194.
doi: 10.1039/C7QO01154G |
| [15] |
Ji, X.; Li, D.; Wang, Z.; Tan, M.; Huang, H.; Deng, G. J. Asian J. Org. Chem. 2018, 7, 711.
doi: 10.1002/ajoc.201800036 |
| [16] |
Xu, M. M.; Cao, W. B.; Xu, X. P.; Ji, S. J. Chem. Commun. 2018, 54, 12602.
doi: 10.1039/C8CC07721E |
| [17] |
Chen, W. L.; Wu, S. Y.; Mo, X. L.; Wei, L. X.; Liang, C.; Mo, D. L. Org. Lett. 2018, 20, 3527.
doi: 10.1021/acs.orglett.8b01294 pmid: 29798675 |
| [18] |
He, J.; Dong, J.; Su, L.; Wu, S.; Liu, L.; Yin, S. F.; Zhou, Y. Org. Lett. 2020, 22, 2522.
doi: 10.1021/acs.orglett.0c00271 |
| [19] |
Llpis, N.; Gisbert, P.; Baeza, A. Adv. Synth. Catal. 2021, 363, 3245.
doi: 10.1002/adsc.202100214 |
| [20] |
Samineni, R.; Bandi, C. R. C.; Srihari, P.; Mehta, G. Org. Lett. 2016, 18, 6184.
pmid: 27934377 |
| [21] |
Wang, M.; Yang, Y.; Song, B.; Yin, L.; Yan, S.; Li, Y. Org. Lett. 2019, 22, 155.
doi: 10.1021/acs.orglett.9b04081 |
| [22] |
Klare, H. F. T.; Goldberg, A. F. G.; Duquette, D. C.; Stoltz, B. M. Org. Lett. 2017, 19, 988.
doi: 10.1021/acs.orglett.6b03789 |
| [23] |
Holzschneider, K.; Mohr, F.; Kirsch, S. F. Org. Lett. 2018, 20, 7066.
doi: 10.1021/acs.orglett.8b03013 pmid: 30365325 |
| [24] |
Ubale, A. S.; Shaikh, M. A.; Gnanaprakasam, B. J. Org. Chem. 2021, 86, 9621.
doi: 10.1021/acs.joc.1c00889 |
| [25] |
Miao, C. B.; Zeng, Y. M.; Shi, T.; Liu, R.; Wei, P. F.; Sun, X. Q.; Yang, H. T. J. Org. Chem. 2016, 81, 43.
doi: 10.1021/acs.joc.5b02054 |
| [26] |
Shao, Y.; Zeng, Y. M.; Ji, J. Y.; Sun, X. Q.; Yang, H. T.; Miao, C. B. J. Org. Chem. 2016, 81, 12443.
doi: 10.1021/acs.joc.6b01993 |
| [27] |
He, Y.; Wang, H.; Xu, L.; Li, D. Y.; Ge, J. H.; Feng, D. F.; Feng, W.; Zou, G.; Liu, P. N. Org. Lett. 2021, 24, 121.
doi: 10.1021/acs.orglett.1c03652 |
| [28] |
Jia, F. C.; Zhou, Z. W.; Xu, C.; Wu, Y. D.; Wu, A. X. Org. Lett. 2016, 18, 2942.
doi: 10.1021/acs.orglett.6b01291 |
| [29] |
Zhou, Z. W.; Jia, F. C.; Xu, C.; Jiang, S. F.; Wu, Y. D.; Wu, A. X. Chem. Commun. 2017, 53, 1056.
doi: 10.1039/C6CC09376K |
| [30] |
Jiang, S. F.; Xu, C.; Zhou, Z. W.; Zhang, Q.; Wen, X. H.; Jia, F. C.; Wu, A. X. Org. Lett. 2018, 20, 4231.
doi: 10.1021/acs.orglett.8b01645 |
| [31] |
Li, P. G.; Yan, C.; Zhu, S.; Liu, S. H.; Zou, L. H. Org. Chem. Front. 2018, 5, 3464.
doi: 10.1039/C8QO01039K |
| [32] |
Jia, F. C.; Chen, T. Z.; Hu, X. Q. Org. Chem. Front. 2020, 7, 1635.
doi: 10.1039/D0QO00345J |
| [33] |
(a) Sun, J.; Tan, Q.; Yang, W.; Liu, B.; Xu, B. Adv. Synth. Catal. 2014, 356, 388.
doi: 10.1002/adsc.201300818 |
|
(b) Liu, M.; Shu, M.; Yao, C.; Yin, G.; Wang, D.; Huang, J. Org. Lett. 2016, 18, 824.
doi: 10.1021/acs.orglett.6b00113 |
|
| [34] |
Prakash, R.; Gogoi, S. Adv. Synth. Catal. 2016, 358, 3046.
doi: 10.1002/adsc.201600516 |
| [35] |
Wang, L. C.; Du, S.; Chen, Z.; Wu, X. F. Org. Lett. 2020, 22, 5567.
doi: 10.1021/acs.orglett.0c01927 pmid: 32610908 |
| [36] |
Wu, H.; Ma, N.; Song, M.; Zhang, G. Chin. Chem. Lett. 2020, 31, 1580.
doi: 10.1016/j.cclet.2019.10.043 |
| [37] |
Shi, R. G.; Wang, X. H.; Liu, R.; Yan, C. G. Chem. Commun. 2016, 52, 6280.
doi: 10.1039/C6CC00525J |
| [38] |
Balwe, S. G.; Jeong, Y. T. Org. Chem. Front. 2018, 5, 1628.
doi: 10.1039/C8QO00071A |
| [1] | Qinggang Mei, Qinghan Li. Recent Progress of Visible Light-Induced the Synthesis of C(3) (Hetero)arylthio Indole Compounds [J]. Chinese Journal of Organic Chemistry, 2024, 44(2): 398-408. |
| [2] | Wenfeng Bei, Jian Pan, Dongmei Ran, Yilin Liu, Zhen Yang, Ruokun Feng. Cobalt-Catalyzed [4+2] Annulation of Indole Carboxamide with Diynes and Monoacetylene: Direct Access to γ-Carbolinones [J]. Chinese Journal of Organic Chemistry, 2023, 43(9): 3226-3238. |
| [3] | Huanqing Li, Zhaohua Chen, Zujia Chen, Qiwen Qiu, Youcai Zhang, Sihong Chen, Zhaoyang Wang. Research Progress in Mercury Ion Fluorescence Probes Based on Organic Small Molecules [J]. Chinese Journal of Organic Chemistry, 2023, 43(9): 3067-3077. |
| [4] | Dandan Sui, Nannan Cen, Ruoqu Gong, Yang Chen, Wenbo Chen. Supporting-Electrolyte-Free Electrochemical Synthesis of Trifluoromethylated Oxindoles in Continuous Flow [J]. Chinese Journal of Organic Chemistry, 2023, 43(9): 3239-3245. |
| [5] | Yi Wang, Jian Zhang, Yangzi Liu, Xiaoyan Luo, Weiping Deng. Palladium-Catalyzed Asymmetric [3+4] Cycloadditions for the Construction of Cyclohepta[b]indoles [J]. Chinese Journal of Organic Chemistry, 2023, 43(8): 2864-2877. |
| [6] | Yingke Feng, He Wang, Mengxing Cui, Ran Sun, Xin Wang, Yang Chen, Lei Li. Visible-Light-Induced Difluoroalkylated Cyclization of Novel Functionalized Aromatic Isocyanides [J]. Chinese Journal of Organic Chemistry, 2023, 43(8): 2913-2925. |
| [7] | Lixing Sun, Tingting Sun, Haiqing Wang, Shufang Wu, Xiaoye Wang, Tianya Liu, Yuchen Zhang. Lewis Acid-Catalyzed [2+4] Cyclization of 3-Alkyl-2-vinylindoles with α,β-Unsaturated N-Sulfonyl Ketimines [J]. Chinese Journal of Organic Chemistry, 2023, 43(6): 2178-2188. |
| [8] | Zeren Sun, Bingxin Zhai, Guangchao He, Hui Shen, Linya Chen, Shan Zhang, Yi Zou, Qihua Zhu, Yungen Xu. Synthesis and Anti-inflammatory Evaluation of Novel 1,2,3-Triazole Derivatives [J]. Chinese Journal of Organic Chemistry, 2023, 43(6): 2143-2155. |
| [9] | Cunjing Miao, Jiaqi Yao. Recent Advances in the Transformation Reactions of Aromatic Nitriles via C—CN Bond Cleavage [J]. Chinese Journal of Organic Chemistry, 2023, 43(4): 1341-1364. |
| [10] | Mingyang Pang, Honghong Chang, Zhang Feng, Juan Zhang. Recent Advances in Transition-Metal-Catalyzed Tandem Dearomatization of Indoles [J]. Chinese Journal of Organic Chemistry, 2023, 43(4): 1271-1291. |
| [11] | Hua Huang, Xin Li, Jianke Su, Qiuling Song. Difluorocarbene-Enabled Synthesis of 3-Substituted-2-oxoindoles from o-Vinylanilines [J]. Chinese Journal of Organic Chemistry, 2023, 43(3): 1146-1156. |
| [12] | Jinxiao Zhao, Tonghui Wei, Sen Ke, Yi Li. Visible Light-Catalyzed Synthesis of Difluoroalkylated Polycyclic Indoles [J]. Chinese Journal of Organic Chemistry, 2023, 43(3): 1102-1114. |
| [13] | Yueling Liu, Xinxin Zhong, Ganbing Zhang. Density Functional Theory Study for Exploring the Mechanisms of the [3+2] Cycloaddition Reactions between 1-R-3-Phenylpropylidenecyclopropane (R=Me/H) and Furfural Catalyzed by Pd(0) [J]. Chinese Journal of Organic Chemistry, 2023, 43(2): 660-667. |
| [14] | Tingting Liu, Yucai Hu, An Shen. Mechanism of Carbon-Carbon Coupling Reactions Catalyzed by Imine-Ligand-Assisted N-Heterocyclic Carbene Palladium Complexes [J]. Chinese Journal of Organic Chemistry, 2023, 43(2): 622-628. |
| [15] | Changyuan Du, Yucai Tang, Jinglin Duan, Biyu Yang, Yupeng He, Qian Zhou, Xuewen Liu. Organic-Dye-Catalyzed Visible-Light-Mediated Alkoxycarbon-ylation of 2-Aryl-N-acryloyl Indoles with Carbazates [J]. Chinese Journal of Organic Chemistry, 2023, 43(12): 4268-4276. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||