Chinese Journal of Organic Chemistry ›› 2023, Vol. 43 ›› Issue (11): 3806-3825.DOI: 10.6023/cjoc202304023 Previous Articles Next Articles
张雨杉a†, 桓臻a†, 杨金东a,*( ), 程津培a,b,c
), 程津培a,b,c
收稿日期:2023-04-19
修回日期:2023-05-22
发布日期:2023-06-26
作者简介:基金资助:
Yushan Zhanga†, Zhen Huana†, Jindong Yanga( ), Jinpei Chenga,b,c
), Jinpei Chenga,b,c
Received:2023-04-19
Revised:2023-05-22
Published:2023-06-26
Contact:
E-mail: About author:Supported by:Share
Yushan Zhang, Zhen Huan, Jindong Yang, Jinpei Cheng. Recent Advances in Hydrogen Transfer Reactivities of N-Heterocyclic Phosphines[J]. Chinese Journal of Organic Chemistry, 2023, 43(11): 3806-3825.
| Hydride donor | Thermodynamic hydricity | Kinetic hydricity | |||
|---|---|---|---|---|---|
| ΔGH–/(kJ•mol–1) | Solvent | N | Solvent | ||
| NaBH4 | 211.0a | MeCN | 14.74 | DMSO | |
| Me3N-BH3 | 306.0a | MeCN | 7.97 | DCM | |
| PhSiH3 | 403.1a | MeCN | 0.06 | DCM | |
| Hantzsch ester | 257.4 | MeCN | 9.00 | DCM | |
| HMo(CO)3Cp* | 268.0 | MeCN | 4.3 | DCM | |
| HW(CO)3Cp | 285.0 | MeCN | 1.7 | DCM | |
| Hydride donor | Thermodynamic hydricity | Kinetic hydricity | |||
|---|---|---|---|---|---|
| ΔGH–/(kJ•mol–1) | Solvent | N | Solvent | ||
| NaBH4 | 211.0a | MeCN | 14.74 | DMSO | |
| Me3N-BH3 | 306.0a | MeCN | 7.97 | DCM | |
| PhSiH3 | 403.1a | MeCN | 0.06 | DCM | |
| Hantzsch ester | 257.4 | MeCN | 9.00 | DCM | |
| HMo(CO)3Cp* | 268.0 | MeCN | 4.3 | DCM | |
| HW(CO)3Cp | 285.0 | MeCN | 1.7 | DCM | |
| Hydride donor | Thermodynamic hydricity | Kinetic hydricity | |||
|---|---|---|---|---|---|
| ΔGH–/(kJ•mol–1) | Solvent | N | Solvent | ||
| NHP-H1 | 142.7a | MeCN | 25.54 | MeCN | |
| NHP-H2 | 173.3a | MeCN | 17.68 | MeCN | |
| NHP-H3 | 177.5a | MeCN | 19.85 | MeCN | |
| NHP-H4 | 186.3a | MeCN | 20.93 | MeCN | |
| NHP-H6 | 159.5a | MeCN | — | — | |
| NHP-H7 | 198.0a | MeCN | 18.74 | MeCN | |
| NHP-H8 | 204.3a | MeCN | 13.46 | MeCN | |
| NHP-H9 | 260.4 | MeCN | 8.46 | MeCN | |
| Hydride donor | Thermodynamic hydricity | Kinetic hydricity | |||
|---|---|---|---|---|---|
| ΔGH–/(kJ•mol–1) | Solvent | N | Solvent | ||
| NHP-H1 | 142.7a | MeCN | 25.54 | MeCN | |
| NHP-H2 | 173.3a | MeCN | 17.68 | MeCN | |
| NHP-H3 | 177.5a | MeCN | 19.85 | MeCN | |
| NHP-H4 | 186.3a | MeCN | 20.93 | MeCN | |
| NHP-H6 | 159.5a | MeCN | — | — | |
| NHP-H7 | 198.0a | MeCN | 18.74 | MeCN | |
| NHP-H8 | 204.3a | MeCN | 13.46 | MeCN | |
| NHP-H9 | 260.4 | MeCN | 8.46 | MeCN | |


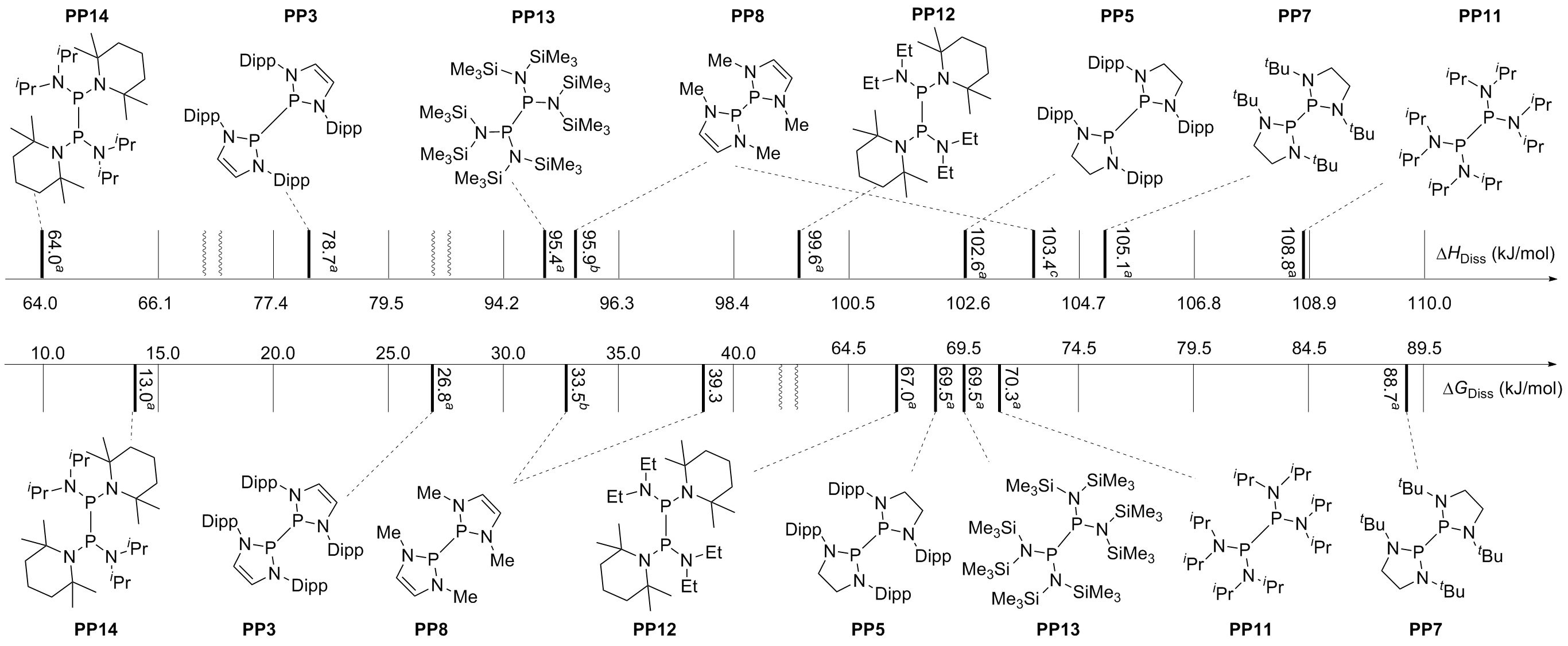
| [1] |
Ai, W.; Zhong, R.; Liu, X.; Liu, Q. Chem. Rev. 2019, 119, 2876.
doi: 10.1021/acs.chemrev.8b00404 |
| [2] |
Jordan, A. J.; Lalic, G.; Sadighi, J. P. Chem. Rev. 2016, 116, 8318.
doi: 10.1021/acs.chemrev.6b00366 |
| [3] |
Roy, M. M. D.; Omaña, A. A.; Wilson, A. S. S.; Hill, M. S.; Aldridge, S.; Rivard, E. Chem. Rev. 2021, 121, 12784.
doi: 10.1021/acs.chemrev.1c00278 |
| [4] |
McGrady, G. S.; Guilera, G. Chem. Soc. Rev. 2003, 32, 383.
doi: 10.1039/b207999m pmid: 14671793 |
| [5] |
Aldridge, S.; Downs, A. J. Chem. Rev. 2001, 101, 3305.
pmid: 11840988 |
| [6] |
Ilic, S.; Alherz, A.; Musgrave, C. B.; Glusac, K. D. Chem. Soc. Rev. 2018, 47, 2809.
doi: 10.1039/C7CS00171A |
| [7] |
Mayr, H.; Patz, M. Angew. Chem., Int. Ed. 1994, 33, 938.
doi: 10.1002/anie.v33:9 |
| [8] |
Mayr, H.; Bug, T.; Gotta, M. F.; Hering, N.; Irrgang, B.; Janker, B.; Kempf, B.; Loos, R.; Ofial, A. R.; Remennikov, G.; Schimmel, H. J. Am. Chem. Soc. 2001, 123, 9500.
pmid: 11572670 |
| [9] |
Wiedner, E. S.; Chambers, M. B.; Pitman, C. L.; Bullock, R. M.; Miller, A. J.; Appel, A. M. Chem. Rev. 2016, 116, 8655.
doi: 10.1021/acs.chemrev.6b00168 pmid: 27483171 |
| [10] |
Brereton, K. R.; Smith, N. E.; Hazari, N.; Miller, A. J. M. Chem. Soc. Rev. 2020, 49, 7929.
doi: 10.1039/D0CS00405G |
| [11] |
Heiden, Z. M.; Lathem, A. P. Organometallics 2015, 34, 1818.
doi: 10.1021/om5011512 |
| [12] |
Sarker, N.; Bruno, J. W. J. Am. Chem. Soc. 1999, 121, 2174.
doi: 10.1021/ja982017b |
| [13] |
Ciancanelli, R.; Noll, B. C.; DuBois, D. L.; DuBois, M. R. J. Am. Chem. Soc. 2002, 124, 2984.
pmid: 11902890 |
| [14] |
Estes, D. P.; Vannucci, A. K.; Hall, A. R.; Lichtenberger, D. L.; Norton, J. R. Organometallics 2011, 30, 3444.
doi: 10.1021/om2001519 |
| [15] |
Hu, Y.; Norton, J. R. J. Am. Chem. Soc. 2014, 136, 5938.
doi: 10.1021/ja412309j |
| [16] |
Horn, M.; Schappele, L. H.; Lang-Wittkowski, G.; Mayr, H.; Ofial, A. R. Chem.-Eur. J. 2013, 19, 249.
doi: 10.1002/chem.v19.1 |
| [17] |
Longeau, A.; Knochel, P. Tetrahedron Lett. 1996, 37, 6099.
doi: 10.1016/0040-4039(96)01296-8 |
| [18] |
Sadow, A. D.; Togni, A. J. Am. Chem. Soc. 2005, 127, 17012.
doi: 10.1021/ja0555163 |
| [19] |
Blum, M.; Kappler, J.; Schlindwein, S. H.; Nieger, M.; Gudat, D. Dalton Trans. 2018, 47, 112.
doi: 10.1039/C7DT04110A |
| [20] |
Leca, D.; Fensterbank, L.; Lacote, E.; Malacria, M. Chem. Soc. Rev. 2005, 34, 858.
doi: 10.1039/b500511f |
| [21] |
Marque, S.; Tordo, P. Top. Curr. Chem. 2005, 250, 43.
|
| [22] |
Gao, Y.; Tang, G.; Zhao, Y. Chin. J. Org. Chem. 2018, 38, 62. (in Chinese)
doi: 10.6023/cjoc201708023 |
|
(高玉珍, 唐果, 赵玉芬, 有机化学, 2018, 38, 62.)
doi: 10.6023/cjoc201708023 |
|
| [23] |
Bezombes, J.-P.; Carré, F.; Chuit, C.; Corriu, R. J. P.; Mehdi, A.; Reyé, C. J. Org. Chem. 1997, 535, 81.
|
| [24] |
Carré, F.; Chuit, C.; Corriu, R. J. P.; Mehdi, A.; Reyé, C. J. Org. Chem. 1997, 529, 59.
|
| [25] |
Gudat, D.; Haghverdi, A.; Nieger, M. Angew. Chem., Int. Ed. 2000, 39, 3084.
doi: 10.1002/(ISSN)1521-3773 |
| [26] |
Fleming, S.; Lupton, M. K.; Jekot, K. Inorg. Chem. 1972, 11, 2534.
doi: 10.1021/ic50116a050 |
| [27] |
Maryanoff, B. E.; Hutchins, R. O. J. Org. Chem 1972, 37, 3475.
doi: 10.1021/jo00795a018 |
| [28] |
Karaghiosoff, K.; Majoral, J. P.; Meriem, A.; Navech, J.; Schmid- peter, A. Tetrahedron Lett. 1983, 24, 2137.
doi: 10.1016/S0040-4039(00)81864-X |
| [29] |
Kibardin, A. M.; Mikhailov, Y. B.; Gryaznova, T. V.; Pudovik, A. N. Bull. Acad. Sci. USSR, Div. Chem. Sci. 1986, 35, 878.
doi: 10.1007/BF00954265 |
| [30] |
Jennings, W. B.; Randall, D.; Worley, S. D.; Hargis, J. H. J. Chem. Soc., Perkin Trans. II 1981, 1411.
|
| [31] |
Dube, J. W.; Farrar, G. J.; Norton, E. L.; Szekely, K. L. S.; Cooper, B. F. T.; Macdonald, C. L. B. Organometallics 2009, 28, 4377.
doi: 10.1021/om900420g |
| [32] |
Burck, S.; Gudat, D.; Nieger, M.; Du Mont, W.-W. J. Am. Chem. Soc. 2006, 128, 3946.
doi: 10.1021/ja057827j |
| [33] |
Alkhater, M. F.; Alherz, A. W.; Musgrave, C. B. Phys. Chem. Chem. Phys. 2021, 23, 17794.
doi: 10.1039/D1CP02193A |
| [34] |
Zhang, J.; Yang, J.-D.; Cheng, J.-P. Angew. Chem., Int. Ed. 2019, 58, 5983.
doi: 10.1002/anie.v58.18 |
| [35] |
Liu, L. L.; Wu, Y.; Chen, P.; Chan, C.; Xu, J.; Zhu, J.; Zhao, Y. Org. Chem. Front. 2016, 3, 423.
doi: 10.1039/C6QO00002A |
| [36] |
Zhang, J.; Yang, J.-D.; Cheng, J.-P. Chem. Sci. 2020, 11, 3672.
doi: 10.1039/C9SC05883D |
| [37] |
Chong, C. C.; Hirao, H.; Kinjo, R. Angew. Chem., Int. Ed. 2014, 53, 3342.
doi: 10.1002/anie.v53.13 |
| [38] |
Waterman, R. Organometallics 2013, 32, 7249.
doi: 10.1021/om400760k |
| [39] |
Chong, C. C.; Hirao, H.; Kinjo, R. Angew. Chem., Int. Ed. 2015, 54, 190.
doi: 10.1002/anie.v54.1 |
| [40] |
Edge, R.; Less, R. J.; McInnes, E. J. L.; Müther, K.; Naseri, V.; Rawson, J. M.; Wright, D. S. Chem. Commun. 2009, 1691.
|
| [41] |
Gudat, D. Dalton Trans. 2016, 45, 5896.
doi: 10.1039/c6dt00085a pmid: 26863391 |
| [42] |
Ould, D. M. C.; Melen, R. L. Chem.-Eur. J. 2020, 26, 9835.
doi: 10.1002/chem.v26.44 |
| [43] |
Speed, A. W. H. Chem. Soc. Rev. 2020, 49, 8335.
doi: 10.1039/D0CS00476F |
| [44] |
Zhang, J.; Yang, J.-D.; Cheng, J.-P. Natl. Sci. Rev. 2021, 8, nwaa253.
doi: 10.1093/nsr/nwaa253 |
| [45] |
Zhang, Y.-S.; Huan, Z.; Yang, J.-D.; Cheng, J.-P. Chem. Commun. 2022, 58, 12528.
doi: 10.1039/D2CC04844B |
| [46] |
Ould, D. M. C.; Tran, T. T. P.; Rawson, J. M.; Melen, R. L. Dalton Trans. 2019, 48, 16922.
doi: 10.1039/C9DT03577J |
| [47] |
Chong, C. C.; Kinjo, R. Angew. Chem., Int. Ed. 2015, 54, 12116.
doi: 10.1002/anie.v54.41 |
| [48] |
Lu, Y.; Gao, Z. H.; Chen, X. Y.; Guo, J.; Liu, Z.; Dang, Y.; Ye, S.; Wang, Z. X. Chem. Sci. 2017, 8, 7637.
doi: 10.1039/C7SC00824D |
| [49] |
Adams, M. R.; Tien, C. H.; Huchenski, B. S. N.; Ferguson, M. J.; Speed, A. W. H. Angew. Chem., Int. Ed. 2017, 56, 6268.
doi: 10.1002/anie.v56.22 |
| [50] |
Chong, C. C.; Rao, B.; Kinjo, R. ACS Catal. 2017, 7, 5814.
doi: 10.1021/acscatal.7b01338 |
| [51] |
Reed, J. H.; Cramer, N. ChemCatChem 2020, 12, 4262.
doi: 10.1002/cctc.v12.17 |
| [52] |
Reed, J. H.; Donets, P. A.; Miaskiewicz, S.; Cramer, N. Angew. Chem., Int. Ed. 2019, 58, 8893.
doi: 10.1002/anie.v58.26 |
| [53] |
Zhang, G.; Cramer, N. Angew. Chem., Int. Ed. 2023, 62, e202301076.
doi: 10.1002/anie.v62.17 |
| [54] |
Miaskiewicz, S.; Reed, J. H.; Donets, P. A.; Oliveira, C. C.; Cramer, N. Angew. Chem., Int. Ed. 2018, 57, 4039.
doi: 10.1002/anie.v57.15 |
| [55] |
Lin, Y. C.; Hatzakis, E.; McCarthy, S. M.; Reichl, K. D.; Lai, T. Y.; Yennawar, H. P.; Radosevich, A. T. J. Am. Chem. Soc. 2017, 139, 6008.
doi: 10.1021/jacs.7b02512 |
| [56] |
Adams, M. R.; Tien, C. H.; McDonald, R.; Speed, A. W. H. Angew. Chem., Int. Ed. 2017, 56, 16660.
doi: 10.1002/anie.v56.52 |
| [57] |
Rao, B.; Chong, C. C.; Kinjo, R. J. Am. Chem. Soc. 2018, 140, 652.
doi: 10.1021/jacs.7b09754 |
| [58] |
Lundrigan, T.; Welsh, E. N.; Hynes, T.; Tien, C. H.; Adams, M. R.; Roy, K. R.; Robertson, K. N.; Speed, A. W. H. J. Am. Chem. Soc. 2019, 141, 14083.
doi: 10.1021/jacs.9b07293 pmid: 31441650 |
| [59] |
Lundrigan, T.; Tien, C. H.; Robertson, K. N.; Speed, A. W. H. Chem. Commun. 2020, 56, 8027.
doi: 10.1039/D0CC01072C |
| [60] |
Hynes, T.; Welsh, E. N.; McDonald, R.; Ferguson, M. J.; Speed, A. W. H. Organometallics 2018, 37, 841.
doi: 10.1021/acs.organomet.8b00028 |
| [61] |
Zhang, J.; Yang, J.-D.; Cheng, J.-P. Nat. Commun. 2021, 12, 2835.
doi: 10.1038/s41467-021-23101-3 |
| [62] |
Zhang, J.; Zhao, X.; Yang, J.-D.; Cheng, J.-P. J. Org. Chem. 2022, 87, 294.
doi: 10.1021/acs.joc.1c02360 |
| [63] |
Puntigam, O.; Förster, D.; Giffin, N. A.; Burck, S.; Bender, J.; Ehret, F.; Hendsbee, A. D.; Nieger, M.; Masuda, J. D.; Gudat, D. Eur. J. Inorg. Chem. 2013, 2041.
|
| [64] |
Burck, S.; Gudat, D.; Nieger, M. Angew. Chem., Int. Ed. 2004, 43, 4801.
doi: 10.1002/anie.v43:36 |
| [65] |
Burck, S.; Götz, K.; Kaupp, M.; Nieger, M.; Weber, J.; Schmedt auf der Günne, J.; Gudat, D. J. Am. Chem. Soc. 2009, 131, 10763.
doi: 10.1021/ja903156p |
| [66] |
Ma, M.; Shen, L.; Wang, H.; Zhao, Y.; Wu, B.; Yang, X.-J. Organometallics 2020, 39, 1440.
doi: 10.1021/acs.organomet.0c00136 |
| [67] |
Abakumov, G. A.; Druzhkov, N. O.; Kazakov, G. G.; Fukin, G. K.; Rumyantsev, R. V.; Cherkasov, V. K. Dokl. Chem. 2019, 489, 279.
doi: 10.1134/S0012500819120012 |
| [68] |
Förster, D.; Dilger, H.; Ehret, F.; Nieger, M.; Gudat, D. Eur. J. Inorg. Chem. 2012, 3989.
|
| [69] |
Blum, M.; Puntigam, O.; Plebst, S.; Ehret, F.; Bender, J.; Nieger, M.; Gudat, D. Dalton Trans. 2016, 45, 1987.
doi: 10.1039/c5dt02854j pmid: 26337501 |
| [70] |
Giffin, N. A.; Hendsbee, A. D.; Masuda, J. D. Dalton Trans. 2016, 45, 12636.
doi: 10.1039/c6dt02790c pmid: 27443569 |
| [71] |
Zhang, J.; Yang, J.-D.; Cheng, J.-P. Chem. Sci. 2020, 11, 4786.
doi: 10.1039/D0SC01352H |
| [72] |
Huchenski, B. S. N.; Robertson, K. N.; Speed, A. W. H. Eur. J. Org. Chem. 2020, 5140.
|
| [73] |
Zhang, J.; Yang, J.-D.; Cheng, J.-P. Chem. Sci. 2020, 11, 8476.
doi: 10.1039/D0SC03220D |
| [74] |
Klett, J.; Wozniak, L.; Cramer, N. Angew. Chem., Int. Ed. 2022, 134, e202202306.
doi: 10.1002/ange.v134.30 |
| [75] |
Riley, R. D.; Huchenski, B. S. N.; Bamford, K. L.; Speed, A. W. H. Angew. Chem., Int. Ed. 2022, 134, e202204088.
doi: 10.1002/ange.v134.30 |
| [1] | Chun-Xia Cheng, Lu-Ping Wu, Feng Sha, Xin-Yan Wu. Enantioselective Vinylogous Allylic Alkylation of Coumarins with Morita-Baylis-Hillman Carbonates Catalyzed by Chiral Phosphine-Amide [J]. Chinese Journal of Organic Chemistry, 2023, 43(9): 3188-3195. |
| [2] | Yiwen Quan, Xinhui Jiang, Wenjun Li, Jian Wang. Access to α-Vinyl β-Alkynyl Enals via an Organocatalytic Deconjugation-Aldol Condensation Sequence [J]. Chinese Journal of Organic Chemistry, 2023, 43(6): 2120-2125. |
| [3] | Ke Jing, Panke Zhang, Senmiao Xu. Application of 1,4-Azaborines in Organic and Transition Metal Catalysis [J]. Chinese Journal of Organic Chemistry, 2023, 43(5): 1742-1750. |
| [4] | Xinyu Zhang, Huihui Geng, Shilei Zhang, Wei Wang, Xiaobei Chen. A Method for the Synthesis of Deuterated Benzoins Catalyzed by N-Heterocyclic Carbene [J]. Chinese Journal of Organic Chemistry, 2023, 43(4): 1510-1516. |
| [5] | Weidi Cao, Xiaohua Liu. Recent Advances on Catalytic Enantioselective Protonation for Construction of α-Tertiary Carbonyl Compounds [J]. Chinese Journal of Organic Chemistry, 2023, 43(3): 961-973. |
| [6] | Ling Meng, Jun Wang. Research Progress on Synthesis of Thioflavonoids [J]. Chinese Journal of Organic Chemistry, 2023, 43(3): 873-891. |
| [7] | Chunbo Dai, Siqi Xia, Xiaoyu Chen, Limin Yang. N-Heterocyclic Carbene (NHC)-Catalyzed [4+3] Cycloaddition to Synthesize 4-Aminobenzoheptenolactons [J]. Chinese Journal of Organic Chemistry, 2023, 43(3): 1084-1090. |
| [8] | Xing Wang, Qianqian Song, Xuling Chen, Pengfei Li, Yunkun Qi, Wenjun Li. Organocatalytic Regio- and Enantioselective aza-1,8-Conjugate Additions of Isoxazol-5(4H)-ones to 6-Methide-6H-indoles [J]. Chinese Journal of Organic Chemistry, 2022, 42(6): 1722-1734. |
| [9] | Zhiwei Ma, Xiaopei Chen, Chuanchuan Wang, Jianling Wang, Jingchao Tao, Quanjian Lü. Chiral Squaramide Catalyzed Enantioselective Michael Addition of Cyclic 1,3-Diketones to β,γ-Unsaturated α-Keto Esters [J]. Chinese Journal of Organic Chemistry, 2022, 42(5): 1520-1526. |
| [10] | Ting Yao, Jiayan Li, Jiaming Wang, Changgui Zhao. Recent Advances for the Construction of Seven-Membered Ring Catalyzed by N-Heterocyclic Carbenes [J]. Chinese Journal of Organic Chemistry, 2022, 42(4): 925-944. |
| [11] | Hongxia Ren, Mengmeng Ma, You Huang. Progress in Synthesis of Nitrogen Heterocycles Catalyzed by Chiral Phosphine [J]. Chinese Journal of Organic Chemistry, 2022, 42(10): 3129-3142. |
| [12] | Huiming Di, Yunting Liu, Yanrong Ma, Xinyue Yang, Hui Jin, Lixin Zhang. Recent Advances in Organocatalytic Asymmetric Synthesis of 3,4-Dihydropyran-2-ones and 3,4-Dihydropyridin-2-ones [J]. Chinese Journal of Organic Chemistry, 2021, 41(6): 2228-2248. |
| [13] | Junwei Zhang, Hao Wu, Weixin Zhang, Liming Wang, Ying Jin. Enantioselective Friedel-Crafts Reaction of Indoles with Isatins Catalyzed by Cinchona Alkaloid Silyl Ether Derivative [J]. Chinese Journal of Organic Chemistry, 2021, 41(3): 1187-1192. |
| [14] | Quanbin Jiang, Xiaodan Zhao. Chiral Bifunctional Chalcogenide-Catalyzed Enantioselective Electrophilic Thiofunctionalization of Alkenes [J]. Chinese Journal of Organic Chemistry, 2021, 41(2): 443-454. |
| [15] | Min Zhao, Fei Li, Yizheng Cheng, Youming Wang, Zhenghong Zhou. Optically Active 3,4-Dihydrocoumarins via Organocatalyzed Asymmetric [4+2] Annulation of ortho-Hydroxyl Functionalized p-Quinone Methides with β-Keto Acylpyrazoles [J]. Chinese Journal of Organic Chemistry, 2021, 41(10): 4039-4049. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||