Chinese Journal of Organic Chemistry ›› 2025, Vol. 45 ›› Issue (8): 2825-2835.DOI: 10.6023/cjoc202502018 Previous Articles Next Articles
ARTICLES
收稿日期:2025-02-18
修回日期:2025-04-18
发布日期:2025-05-06
基金资助:
Xinghui Tao, Kaiyue Guo, Jitan Zhang, Meihua Xie*( ), Jiaping Wu*(
), Jiaping Wu*( )
)
Received:2025-02-18
Revised:2025-04-18
Published:2025-05-06
Contact:
*E-mail:wujiaping@ahnu.edu.cn;xiemh@mail.ahnu.edu.cn
Supported by:Share
Xinghui Tao, Kaiyue Guo, Jitan Zhang, Meihua Xie, Jiaping Wu. Synthesis of Functionalized Azepinones and Phenanthridines via a Pd-Catalyzed ortho-C—H Amination/C—H Arylation Cyclization Cascade of Aryl Bromides[J]. Chinese Journal of Organic Chemistry, 2025, 45(8): 2825-2835.
| Entry | [Pd] | Base | Ligand | Solvent | Temp./℃ | Yieldb/% |
|---|---|---|---|---|---|---|
| 1 | Pd(OAc)2 | Cs2CO3 | PPh3 | Toluene | 80 | 23 |
| 2 | Pd(PPh3)4 | Cs2CO3 | PPh3 | Toluene | 80 | 31 |
| 3 | Pd2(dba)3 | Cs2CO3 | PPh3 | Toluene | 80 | 10 |
| 4 | Pd(TFA)2 | Cs2CO3 | PPh3 | Toluene | 80 | 43 |
| 5 | PdCl2(PPh3)2 | Cs2CO3 | PPh3 | Toluene | 80 | 21 |
| 6 | Pd(TFA)2 | K3PO4 | PPh3 | Toluene | 80 | 12 |
| 7 | Pd(TFA)2 | t-BuOK | PPh3 | Toluene | 80 | 15 |
| 8 | Pd(TFA)2 | K2CO3 | PPh3 | Toluene | 80 | 10 |
| 9 | Pd(TFA)2 | Cs2CO3 | P(o-MeOC6H4)3 | Toluene | 80 | 59 |
| 10 | Pd(TFA)2 | Cs2CO3 | S-Phos | Toluene | 80 | 23 |
| 11 | Pd(TFA)2 | Cs2CO3 | DPPB | Toluene | 80 | 18 |
| 12 | Pd(TFA)2 | Cs2CO3 | DPPE | Toluene | 80 | 14 |
| 13 | Pd(TFA)2 | Cs2CO3 | P(o-MeOC6H4)3 | DME | 80 | 72 |
| 14 | Pd(TFA)2 | Cs2CO3 | P(o-MeOC6H4)3 | THF | 80 | 32 |
| 15 | Pd(TFA)2 | Cs2CO3 | P(o-MeOC6H4)3 | DMF | 80 | 29 |
| 16 | Pd(TFA)2 | Cs2CO3 | P(o-MeOC6H4)3 | Dioxane | 80 | 40 |
| 17 | Pd(TFA)2 | Cs2CO3 | P(o-MeOC6H4)3 | o-Xylene | 80 | 17 |
| 18 | Pd(TFA)2 | Cs2CO3 | P(o-MeOC6H4)3 | DME | 60 | 53 |
| 19 | Pd(TFA)2 | Cs2CO3 | P(o-MeOC6H4)3 | DME | 90 | 65 |
| 20 | Pd(TFA)2 | Cs2CO3 | P(o-MeOC6H4)3 | DME | 100 | 63 |
| 21c | Pd(TFA)2 | Cs2CO3 | P(o-MeOC6H4)3 | DME | 80 | 88 |
| Entry | [Pd] | Base | Ligand | Solvent | Temp./℃ | Yieldb/% |
|---|---|---|---|---|---|---|
| 1 | Pd(OAc)2 | Cs2CO3 | PPh3 | Toluene | 80 | 23 |
| 2 | Pd(PPh3)4 | Cs2CO3 | PPh3 | Toluene | 80 | 31 |
| 3 | Pd2(dba)3 | Cs2CO3 | PPh3 | Toluene | 80 | 10 |
| 4 | Pd(TFA)2 | Cs2CO3 | PPh3 | Toluene | 80 | 43 |
| 5 | PdCl2(PPh3)2 | Cs2CO3 | PPh3 | Toluene | 80 | 21 |
| 6 | Pd(TFA)2 | K3PO4 | PPh3 | Toluene | 80 | 12 |
| 7 | Pd(TFA)2 | t-BuOK | PPh3 | Toluene | 80 | 15 |
| 8 | Pd(TFA)2 | K2CO3 | PPh3 | Toluene | 80 | 10 |
| 9 | Pd(TFA)2 | Cs2CO3 | P(o-MeOC6H4)3 | Toluene | 80 | 59 |
| 10 | Pd(TFA)2 | Cs2CO3 | S-Phos | Toluene | 80 | 23 |
| 11 | Pd(TFA)2 | Cs2CO3 | DPPB | Toluene | 80 | 18 |
| 12 | Pd(TFA)2 | Cs2CO3 | DPPE | Toluene | 80 | 14 |
| 13 | Pd(TFA)2 | Cs2CO3 | P(o-MeOC6H4)3 | DME | 80 | 72 |
| 14 | Pd(TFA)2 | Cs2CO3 | P(o-MeOC6H4)3 | THF | 80 | 32 |
| 15 | Pd(TFA)2 | Cs2CO3 | P(o-MeOC6H4)3 | DMF | 80 | 29 |
| 16 | Pd(TFA)2 | Cs2CO3 | P(o-MeOC6H4)3 | Dioxane | 80 | 40 |
| 17 | Pd(TFA)2 | Cs2CO3 | P(o-MeOC6H4)3 | o-Xylene | 80 | 17 |
| 18 | Pd(TFA)2 | Cs2CO3 | P(o-MeOC6H4)3 | DME | 60 | 53 |
| 19 | Pd(TFA)2 | Cs2CO3 | P(o-MeOC6H4)3 | DME | 90 | 65 |
| 20 | Pd(TFA)2 | Cs2CO3 | P(o-MeOC6H4)3 | DME | 100 | 63 |
| 21c | Pd(TFA)2 | Cs2CO3 | P(o-MeOC6H4)3 | DME | 80 | 88 |
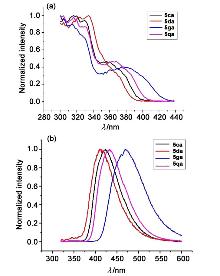

| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
doi: 10.1021/ja410823e pmid: 24256439 |
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
doi: 10.1021/acs.orglett.6b02249 pmid: 27551772 |
| [13] |
doi: 10.1021/acs.orglett.6b02113 pmid: 27551884 |
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
doi: 10.1021/acs.joc.0c00475 pmid: 32441518 |
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
|
| [31] |
doi: 10.1021/ja206229y pmid: 21838278 |
| [32] |
|
| [33] |
|
| [34] |
|
| [35] |
|
|
(王玉斌, 郭成, 陶晟, 刘纪昌, 赵基钢, 刘宁, 代斌, 有机化学, 2022, 42, 1146.)
doi: 10.6023/cjoc202107062 |
|
| [36] |
pmid: 11841870 |
| [37] |
|
| [38] |
|
| [39] |
|
| [40] |
|
| [41] |
|
| [1] | Chunjie Qian, Boyang He, Xingyu Liu, Ping Wang, Guangchun Gao, Shihui Liu. Copper/Selectfluor Co-catalyzed Ritter-Type Benzylic C—H Amination [J]. Chinese Journal of Organic Chemistry, 2025, 45(6): 2181-2188. |
| [2] | Mingshun Mei, Yanghui Zhang. Palladium-Catalyzed Intermolecular Functionalization of Unactivated Methylene C(sp3)—H Bonds [J]. Chinese Journal of Organic Chemistry, 2025, 45(2): 620-640. |
| [3] | Yu Zou, Weicong Guo, Jun Wang. Application of Planar Chiral Cyclopentadienyl Rhodium Catalysts without Chiral Substituents in Asymmetric C—H Activation [J]. Chinese Journal of Organic Chemistry, 2025, 45(2): 466-476. |
| [4] | Xuan Wang, Maochen Liu, Yao Zhong, Renjie Song. Advances in Design of Covalent Organic Frameworks and Their Application in Catalytic C—H Activation [J]. Chinese Journal of Organic Chemistry, 2025, 45(2): 448-465. |
| [5] | Zeshui Liu, Zhenzhen Guo, Junlong Niu. Recent Progress in the Construction of Silacycles by Transition- Metal-Catalyzed C—H Silylation [J]. Chinese Journal of Organic Chemistry, 2025, 45(2): 423-447. |
| [6] | Fan Yang, Xiaomeng Fan, Xuejing Yao, Ruijie Mi, Songjie Yu, Xingwei Li, Jian Xiao. Rhodium(III)-Catalyzed Annulative Coupling between Sulfoxonium Ylides and Diazo Compounds [J]. Chinese Journal of Organic Chemistry, 2025, 45(1): 331-342. |
| [7] | Mengke Wen, Yiyue Li, Haokang Du, Zhangpei Chen, Xifa Yang. Synthesis of Spiropyrans via Rh(III)-Catalyzed [3+3] Cyclization of 3-Aryl-2H-1,4-benzoxazine and Diazonaphthalene-2H-ones [J]. Chinese Journal of Organic Chemistry, 2024, 44(7): 2223-2232. |
| [8] | Kejin Huang, Jinbo Cai, Ruige Wang, Yonghong Zhang, Bin Wang, Yu Xia, Weiwei Jin, Xinyong Li, Chenjiang Liu. Electrochemical C(sp2)—H Bromination of Glycine Derivatives Enabled by Boron [J]. Chinese Journal of Organic Chemistry, 2024, 44(3): 989-996. |
| [9] | Zile Zhu, Pengfei Li, Youai Qiu. Recent Advance in Electrochemical C(sp2)—H Amination of Arenes [J]. Chinese Journal of Organic Chemistry, 2024, 44(3): 871-891. |
| [10] | Sifan Dong, Haolong Li, Yuan Qin, Shiming Fan, Shouxin Liu. Research Progress of Amino Acids as Transient Directing Groups in C—H Bond Activation Reactions [J]. Chinese Journal of Organic Chemistry, 2023, 43(7): 2351-2367. |
| [11] | Xiaojing Hu, Feixiang Guo, Runqing Zhu, Bingqi Zhou, Tao Zhang, Lizhen Fang. Synthesis of p-Alkoxy Phenol and Its Application after Dearomatization [J]. Chinese Journal of Organic Chemistry, 2023, 43(6): 2239-2244. |
| [12] | Cheng Yuan, Changduo Pan. Recent Advances in the N-Aryl C—H Functionalization Using 7-Azaindole as Intrinsic Directing Group [J]. Chinese Journal of Organic Chemistry, 2023, 43(1): 156-170. |
| [13] | Mingzhou Shang, Lanlan Zhang, Miaomiao Chen, Wangcheng Hu, Xinwei He, Hongjian Lu. Synthesis of Esterified/Fused Isocoumarins via Rh-Catalyzed C—H Activation/Transannulative Coupling/Annulation of Phthalic Anhydrides with Cyclic 2-Diazo-1,3-diketones and Methanol [J]. Chinese Journal of Organic Chemistry, 2022, 42(11): 3816-3823. |
| [14] | Shangfei Huo, Hong Chen, Weiwei Zuo. Selective Chlorination of Methane Photochemically Mediated by Ferric Chloride at Ambient Temperature [J]. Chinese Journal of Organic Chemistry, 2021, 41(4): 1683-1690. |
| [15] | Luhua Liu, Rongrong Du, Senmiao Xu. Ligand-Free Iridium-Catalyzed Borylation of Secondary Benzylic C—H Bonds [J]. Chinese Journal of Organic Chemistry, 2021, 41(4): 1572-1581. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||