Chinese Journal of Organic Chemistry ›› 2025, Vol. 45 ›› Issue (12): 4375-4383.DOI: 10.6023/cjoc202501004 Previous Articles Next Articles
ARTICLES
收稿日期:2025-04-10
修回日期:2025-05-25
发布日期:2025-08-18
通讯作者:
王敏
基金资助:
Ruijie Yang, Xin Wang, Zhihui Zhu, Yiming Xu, Zhiguo Song, Min Wang*( )
)
Received:2025-04-10
Revised:2025-05-25
Published:2025-08-18
Contact:
Min Wang
Supported by:Share
Ruijie Yang, Xin Wang, Zhihui Zhu, Yiming Xu, Zhiguo Song, Min Wang. Green Synthesis of 2-Substituted-2,3-dihydroquinazolin-4(1H)-ones Catalyzed by a Novel Zn(II) Complex[J]. Chinese Journal of Organic Chemistry, 2025, 45(12): 4375-4383.
| Parameter | Value |
|---|---|
| Formula | C44H68ZnN12O24S4 |
| Formula weight | 1314.5 |
| Crystal system | Triclinic |
| Space group | P-1 |
| a/nm | 0.75461(3) |
| b/nm | 0.91215(3) |
| c/nm | 1.24011(10) |
| α/(°) | 75.1310(10) |
| β/(°) | 89.0470(10) |
| γ/(°) | 78.3130(10) |
| V/nm3 | 0.80734(5) |
| Z | 1 |
| Dc/(mg•m-3) | 1.522 |
| Goodness-of-fit F2 | 1.100 |
| R1a, wR2b | 0.0497, 0.1492 |
| R1, wR2 (all data) | 0.0522, 0.1520 |
| Parameter | Value |
|---|---|
| Formula | C44H68ZnN12O24S4 |
| Formula weight | 1314.5 |
| Crystal system | Triclinic |
| Space group | P-1 |
| a/nm | 0.75461(3) |
| b/nm | 0.91215(3) |
| c/nm | 1.24011(10) |
| α/(°) | 75.1310(10) |
| β/(°) | 89.0470(10) |
| γ/(°) | 78.3130(10) |
| V/nm3 | 0.80734(5) |
| Z | 1 |
| Dc/(mg•m-3) | 1.522 |
| Goodness-of-fit F2 | 1.100 |
| R1a, wR2b | 0.0497, 0.1492 |
| R1, wR2 (all data) | 0.0522, 0.1520 |
| Bond | Length/nm | Bond angle | Angle/(°) |
|---|---|---|---|
| Zn(1)—N(1) | 0.2101(3) | O(2W)—Zn(1)—O(1W) | 88.82(11) |
| Zn(1)—N(1)#1 | 0.2101(3) | O(2W)#1—Zn(1)—O(1W) | 91.18(11) |
| Zn(1)—O(1W) | 0.2142(3) | N(1)#1—Zn(1)—O(2W)#1 | 88.63(10) |
| Zn(1)—O(1W)#1 | 0.2142(3) | N(1)#1—Zn(1)—O(1W)#1 | 91.23(11) |
| Zn(1)—O(2W) | 0.2105(2) | O(2W)—Zn(1)—O(1W)#1 | 91.18(11) |
| Zn(1)—O(1W)#1 | 0.2105(2) | N(1)—Zn(1)—O(2W) | 88.63(10) |
| Bond | Length/nm | Bond angle | Angle/(°) |
|---|---|---|---|
| Zn(1)—N(1) | 0.2101(3) | O(2W)—Zn(1)—O(1W) | 88.82(11) |
| Zn(1)—N(1)#1 | 0.2101(3) | O(2W)#1—Zn(1)—O(1W) | 91.18(11) |
| Zn(1)—O(1W) | 0.2142(3) | N(1)#1—Zn(1)—O(2W)#1 | 88.63(10) |
| Zn(1)—O(1W)#1 | 0.2142(3) | N(1)#1—Zn(1)—O(1W)#1 | 91.23(11) |
| Zn(1)—O(2W) | 0.2105(2) | O(2W)—Zn(1)—O(1W)#1 | 91.18(11) |
| Zn(1)—O(1W)#1 | 0.2105(2) | N(1)—Zn(1)—O(2W) | 88.63(10) |
| D—H…A | Bond length (D—H)/nm | Bond length (H…A)/nm | Bond length (D…A)/nm | ∠DHA/(°) |
|---|---|---|---|---|
| O(1W)—H(1WA)…O(1) | 0.085 | 0.220 | 0.2793(4) | 150.9 |
| O(1W)—H(1WB)…O(1)#4 | 0.085 | 0.197 | 0.2809(4) | 167.0 |
| O(2W)—H(2WA)…O(4)#4 | 0.085 | 0.196 | 0.2769(4) | 158.9 |
| O(2W)—H(2WB)…N(3)#5 | 0.085 | 0.205 | 0.2870(4) | 161.7 |
| D—H…A | Bond length (D—H)/nm | Bond length (H…A)/nm | Bond length (D…A)/nm | ∠DHA/(°) |
|---|---|---|---|---|
| O(1W)—H(1WA)…O(1) | 0.085 | 0.220 | 0.2793(4) | 150.9 |
| O(1W)—H(1WB)…O(1)#4 | 0.085 | 0.197 | 0.2809(4) | 167.0 |
| O(2W)—H(2WA)…O(4)#4 | 0.085 | 0.196 | 0.2769(4) | 158.9 |
| O(2W)—H(2WB)…N(3)#5 | 0.085 | 0.205 | 0.2870(4) | 161.7 |
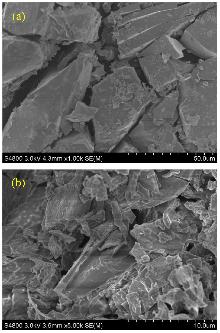

| Entry | R | LUMO/a.u. | Yield/% | m.p./℃ | |
|---|---|---|---|---|---|
| Found | Reported | ||||
| 1 | H | -0.06291 | 92.5, 91.7, 90.8b | 219~222 | 219~222[ |
| 2 | 2-NO2 | -0.10687 | 95.2 | 190~191 | 190~192[ |
| 3 | 2-Cl | -0.07927 | 93.3 | 203~205 | 202~20422] |
| 4 | 2-Br | -0.07660 | 91.8 | 179~181 | 179~182[ |
| 5 | 3-NO2 | -0.10429 | 94.7 | 201~203 | 200~203[ |
| 6 | 3-Cl | -0.07463 | 92.1 | 184~186 | 186~187[ |
| 7 | 3-Br | -0.07444 | 91.6 | 226~228 | 227~228[ |
| 8 | 3-CH3 | -0.06000 | 88.1 | 204~206 | 204[ |
| 9 | 3-CH3O | -0.05980 | 86.9 | 156~158 | 157~158[ |
| 10 | 3-OH | -0.05253 | 86.2 | 224~226 | 223~225[ |
| 11 | 4-NO2 | -0.11459 | 97.3 | 198~200 | 198~200[ |
| 12 | 4-Cl | -0.07305 | 91.7 | 199~202 | 198~201[ |
| 13 | 4-Br | -0.07019 | 90.8 | 194~197 | 195~197[ |
| 14 | 4-CH3 | -0.05901 | 86.7 | 232~234 | 233~234[ |
| 15 | 4-CH3O | -0.05166 | 85.9 | 189~191 | 188~190[ |
| 16 | 4-OH | -0.05108 | 84.5 | 184 | 182[ |
| Entry | R | LUMO/a.u. | Yield/% | m.p./℃ | |
|---|---|---|---|---|---|
| Found | Reported | ||||
| 1 | H | -0.06291 | 92.5, 91.7, 90.8b | 219~222 | 219~222[ |
| 2 | 2-NO2 | -0.10687 | 95.2 | 190~191 | 190~192[ |
| 3 | 2-Cl | -0.07927 | 93.3 | 203~205 | 202~20422] |
| 4 | 2-Br | -0.07660 | 91.8 | 179~181 | 179~182[ |
| 5 | 3-NO2 | -0.10429 | 94.7 | 201~203 | 200~203[ |
| 6 | 3-Cl | -0.07463 | 92.1 | 184~186 | 186~187[ |
| 7 | 3-Br | -0.07444 | 91.6 | 226~228 | 227~228[ |
| 8 | 3-CH3 | -0.06000 | 88.1 | 204~206 | 204[ |
| 9 | 3-CH3O | -0.05980 | 86.9 | 156~158 | 157~158[ |
| 10 | 3-OH | -0.05253 | 86.2 | 224~226 | 223~225[ |
| 11 | 4-NO2 | -0.11459 | 97.3 | 198~200 | 198~200[ |
| 12 | 4-Cl | -0.07305 | 91.7 | 199~202 | 198~201[ |
| 13 | 4-Br | -0.07019 | 90.8 | 194~197 | 195~197[ |
| 14 | 4-CH3 | -0.05901 | 86.7 | 232~234 | 233~234[ |
| 15 | 4-CH3O | -0.05166 | 85.9 | 189~191 | 188~190[ |
| 16 | 4-OH | -0.05108 | 84.5 | 184 | 182[ |
| Atom | Charge | Atom | Charge |
|---|---|---|---|
| Zn | 0.696 | N1 | -0.310 |
| S | 1.245 | N2 | -0.156 |
| O1 | -0.520 | N3 | -0.180 |
| O2 | -0.525 | N4 | -0.171 |
| O3 | -0.243 | N5 | -0.170 |
| O4 | -0.072 | N6 | -0.276 |
| Atom | Charge | Atom | Charge |
|---|---|---|---|
| Zn | 0.696 | N1 | -0.310 |
| S | 1.245 | N2 | -0.156 |
| O1 | -0.520 | N3 | -0.180 |
| O2 | -0.525 | N4 | -0.171 |
| O3 | -0.243 | N5 | -0.170 |
| O4 | -0.072 | N6 | -0.276 |
| [1] |
doi: 10.1002/slct.v2.23 |
| [2] |
|
| [3] |
doi: 10.3390/app12052710 |
| [4] |
doi: 10.1002/jhet.v55.3 |
| [5] |
doi: 10.3390/12122621 |
| [6] |
doi: 10.1016/j.cclet.2010.03.022 |
| [7] |
doi: 10.1016/j.tetlet.2012.10.029 |
| [8] |
doi: 10.1007/s11164-013-1071-x |
| [9] |
doi: 10.1016/j.tetlet.2015.06.004 |
| [10] |
doi: 10.1016/j.tetlet.2019.151587 |
| [11] |
doi: 10.33263/LIANBS |
| [12] |
doi: 10.1016/j.poly.2018.09.002 |
| [13] |
doi: 10.3390/cryst13121681 |
| [14] |
doi: 10.1016/j.jssc.2021.122610 |
| [15] |
doi: 10.1016/j.apsusc.2022.153250 |
| [16] |
doi: 10.1016/j.poly.2018.03.006 |
| [17] |
doi: 10.1016/j.ccr.2010.10.038 |
| [18] |
doi: 10.1039/c3ce41157e |
| [19] |
|
|
(朱为宏, 杨雪艳, 李晶, 有机波谱及性能分析法, 化学工业出版社, 北京 2007, p. 55.)
|
|
| [20] |
doi: 10.1002/ajoc.v1.4 |
| [21] |
|
| [22] |
doi: 10.1007/s11164-016-2634-4 |
| [23] |
|
|
(谢宗波, 张士国, 姜国芳, 梁萌, 乐长高, 有机化学, 2017, 32, 14.)
|
|
| [24] |
|
|
(段建凤, 穆小静, 周曌, 毕旌富, 肖尚友, 化学通报, 2018, 81, 1023.)
|
|
| [25] |
doi: 10.1016/j.tet.2024.133915 |
| [26] |
doi: 10.6023/cjoc201903025 |
|
(张晓鹏, 朱妍洁, 朱奕崧, 李政伟, 张贵生, 有机化学, 2019, 39, 2392.)
doi: 10.6023/cjoc201903025 |
|
| [27] |
doi: 10.1039/C8RA07256F |
| [28] |
|
| [29] |
doi: 10.1107/S0021889808042726 |
| [30] |
|
| [1] | Jidong Zhang, Yao Yang, Jie Zhang, Wei She. Detection of Zn(II) by Tetraphenylethyene Fluorescent Probe Based on Aggregation-Induced Emission (AIE)-Excited State Intramolecular Proton Transfer (ESIPT) Effect [J]. Chinese Journal of Organic Chemistry, 2024, 44(4): 1337-1342. |
| [2] | Yan Dang, Chaohong Jia, Yalan Wang, Li Wang, Yafei Li, Yahong Li. Synthesis and Characterization of Zinc, Lithium and Magnesium Complexes Containing Pyrrolyl Ligands, and Utilization as Catalysts in Borylation of Aryl Iodides and Hydroboration of Aldehydes and Ketones [J]. Chinese Journal of Organic Chemistry, 2023, 43(3): 1124-1135. |
| [3] | Zehui Li, Haoyu Zou, Lincai Li, Yiling Zhao, Hongping Zhu. Synthesis and Propylene Oxide Carbonylation Hydroesterification Catalytic Property of N,O-Ligand Coordination Cobalt Compounds [J]. Chinese Journal of Organic Chemistry, 2023, 43(11): 3907-3915. |
| [4] | Siyi Ding, Weisai Zu, Zongcheng Miao, Liang Xu. Synthetic and Computational Study of Four-Coordinate B,B-Diaryl 8-Aminoquinolate Complexes [J]. Chinese Journal of Organic Chemistry, 2022, 42(3): 812-818. |
| [5] | Qi Lianshan, Wang Tao, Wei Yongmei, Tian Hengshui. Study on the Effect of Co-reductant Aldehydes on Epoxidation of Propylene Catalyzed by Metalloporphyrins [J]. Chinese Journal of Organic Chemistry, 2020, 40(5): 1305-1309. |
| [6] | Zhu Jihua, Zhang Hao, Liu Min, Liu Jingjiang, Liao Yuan, Quan Zhengjun, Wang Xicun. An Intramolecular Charge Transfer (ICT)-Based Fluorescent Probe of Hydrogen Sulphide under pH Control Strategy [J]. Chinese Journal of Organic Chemistry, 2020, 40(4): 1043-1049. |
| [7] | Shen Jiejie, Mao Xuming, Chen Xin'ai, Li Yongquan. Recent Advances in Acyltransferase Domain of Type I Polyktide Synthases [J]. Chin. J. Org. Chem., 2018, 38(9): 2377-2385. |
| [8] | Jin Wenbing, Yuan Hua, Tang Gongli. Strategies for Construction of Cyclopropanes in Natural Products [J]. Chin. J. Org. Chem., 2018, 38(9): 2324-2334. |
| [9] | Zhang Yiwei, Chen Yilin, Fang Xiaolong, Yuan Youzhu, Zhu Hongping. Advances for the Ruthenium Complexes-Based Homogeneous Catalytic Hydrogenation of Oxalates to Ethylene Glycol [J]. Chin. J. Org. Chem., 2017, 37(9): 2275-2286. |
| [10] | Zou Xiaochuan, Shi Kaiyun, Li Jun, Wang Yue, Wang Cun, Deng Chaofang, Ren Yanrong, Tan Jun, Fu Xiangkai. Research Progress on Epoxidation of Olefins Catalyzed by Mn(II, III, V) in Different Valence States [J]. Chin. J. Org. Chem., 2016, 36(8): 1765-1778. |
| [11] | Su Biyun, Jia Peiyu, Wang Yanzhao, Li Yaning, Huang He, Li Qianding. Copolymerization of Ethylene/Polar Monomer Catalyzed by Phosphinoarenesulfonate (PO) Metal Catalysts and the Catalytic Mechanism [J]. Chin. J. Org. Chem., 2016, 36(10): 2344-2352. |
| [12] | Liu Rui, Xiao Shumeng, Zhong Xianghong, Cao Yucai, Liang Shengbiao, Liu Zhenyu, Ye Xiaofeng, Shen An, Zhu Hongping. Advances in Selective Ethylene Oligomerization Based on [PNP]-Ligand Chromium Catalysts [J]. Chin. J. Org. Chem., 2015, 35(9): 1861-1888. |
| [13] | Wang Biyu, Ni Peizhong, Fan Jili, Zheng Huidong, Zhao Suying, Bai Zhengshuai. Pd-Complex Catalyzed Suzuki Cross-Coupling Reaction of Brominated Salicylaldehyde with Pyridylboronic Acid [J]. Chin. J. Org. Chem., 2014, 34(12): 2471-2477. |
| [14] | Xiao Liwei, Jing Xuemin, Kong jie. Synthesis and Catalytic Behaviors toward Styrene Polymerization of Zinc Complexes Bearing 6-Benzimidazolyl-2-aryliminoethyl Pyridine [J]. Chin. J. Org. Chem., 2013, 33(08): 1828-1833. |
| [15] | Huo Yanping, Dai Lili, Huang Baohua, Zhang Kun, Fang Yanxiong, Liu Jun . Synthesis and Photophysics of Novel Zn Metal Complexes Based on 2-Substituted-8-hydroxyquinoline with Nitrobenzene Groups [J]. Chin. J. Org. Chem., 2012, (03): 538-543. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||