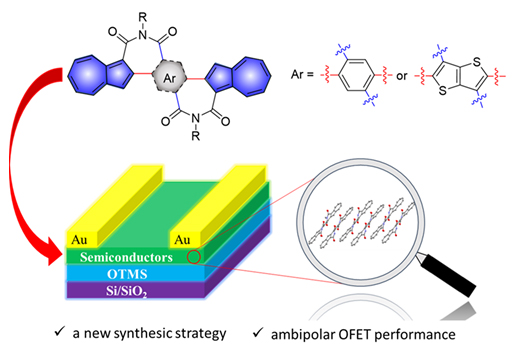
Azulene, a bicyclic nonbenzenoid aromatic hydrocarbon, shows completely different physicochemical properties compared with its isomeric naphthalene. Herein, we made use of the diverse reactivity of each position on azulene to design a new synthetic strategy for azulene-based diimides bridged by phenyl or thieno[3,2-b]thiophenyl group, 2-(azulen-2'-yl)-5-(azulen-2''-yl)benzene-1,1':4,1''-tetracarboxylic diimides (AzAzBDI-1/2) and 2-(azulen-2'-yl)-5- (azulen-2''-yl)thieno[3,2-b]thiophene-3,1':6,1''-tetracarboxylic diimide (AzAzTTDI). The key step was double trifluoroacetylation at 1-position of two azulene moieties of the molecule followed by hydrolysis, anhydridization and imidization to obtain the target compounds. The single crystal structure analysis demonstrates that AzAzBDI-2 has twisted molecular backbone. The adjacent two molecules form a dimer through the intermolecular π-π stacking (0.365 nm) between the five-membered ring and the seven-membered ring of two different azulene units. Strong π-π intermolecular interactions (0.355 nm) exist among the dimers to form a slipped one-dimensional (1D) packing motif in the crystal. For three compounds, the optoelectronic properties were investigated by UV-vis absorption spectra and cyclic voltammetry, and their energy levels of highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO) and the energy gaps were calculated. The HOMO/LUMO energy levels of AzAzBDI-1, AzAzBDI-2 and AzAzTTDI are -5.56/-3.28 eV, -5.56/ -3.30 eV and -5.57/-3.42 eV, respectively. The end absorptions of AzAzBDI-1, AzAzBDI-2 and AzAzTTDI in thin films show obvious red-shift (13, 13 and 25 nm) relative to those in CHCl3 solution, indicating strong intermolecular interactions in solid state. The charge carrier transport properties of three compounds were studied through organic field-effect transistors (OFETs). Bottom-gate and top-contact OFET devices of AzAzBDI-1, AzAzBDI-2 and AzAzTTDI were fabricated by spin-coated their respective solution on octadecyltrimethoxysilane (OTMS)-treated SiO2/Si substrates. Under nitrogen atmosphere, all of these three compounds displayed electron-dominated ambipolar organic semiconductor characteristics. The
| electron mobilities of AzAzBDI-1 and AzAzBDI-2 were 0.068 cm2·V-1·s-1 and 0.086 cm2·V-1·s-1 and the hole mobility were 3.1×10-4 cm2·V-1·s-1 and 1.5×10-3 cm2·V-1·s-1, respectively. OFETs based on AzAzTTDI showed the highest electron mobility and hole mobilities of 0.087 cm2·V-1·s-1 and 8.8×10-3 cm2·V-1·s-1, respectively. The X-ray diffraction (XRD) and atomic force microscopy (AFM) studies demonstrate thin films of AzAzBDI-1, AzAzBDI-2 and AzAzTTDI show better crystallinity and form larger size of microstructures by annealing, which is consistent with the enhanced device performance after thermal annealing.
Key words:
azulene,
nonbenzenoid aromatic hydrocarbon,
imides,
organic field-effect transistors,
organic semiconductor
引用此文
侯斌, 李晶, 辛涵申, 杨笑迪, 高洪磊, 彭培珍, 高希珂. 芳基桥联的二薁二酰亚胺的设计合成及场效应晶体管性能研究[J]. 化学学报, 2020, 78(8): 788-796.
Hou Bin, Li Jing, Xin Hanshen, Yang Xiaodi, Gao Honglei, Peng Peizhen, Gao Xike. Design, Synthesis and Field Effect Characteristics of Diazulene Diimides Bridged by Aromatic Group[J]. Acta Chimica Sinica, 2020, 78(8): 788-796.
导出引用 EndNote|Reference Manager|ProCite|BibTeX|RefWorks
[1] (a) Wheland, G. W.; Mann, D. E. J. Chem. Phys. 1949, 17, 264. (b) Anderson, A. G.; Steckler, B. M. J. Am. Chem. Soc. 1959, 81, 4941.
[2] Michl, J.; Thulstrup, E. W. Tetrahedron 1976, 32, 205.
[3] Beer, M.; Longuet-Higgins, H. C. J. Chem. Phys. 1955, 23, 1390.
[4] Kasha, M. Faraday Soc. 1950, 9, 14.
[5] (a) Wang, D.; Dong, Z.; Xu, J.; Li, D. Chin. J. Org. Chem. 2013, 33, 1559 (in Chinese). (王道林, 董哲, 徐姣, 李帝, 有机化学, 2013, 33, 1559.) (b) Yang, F.; Wang, H.; Zhang, L.; Shi, W.; Zhang, P.; Li, Y.; Yin, S. Chin. J. Org. Chem. 2011, 31, 2106 (in Chinese). (杨芳, 王海峰, 张露昀, 石万棋, 张萍, 李颖, 尹述凡, 有机化学, 2011, 31, 2106.) (c) Wang, D.; Han, S.; Huang, X.; Gu, Z. Chin. J. Org. Chem. 2009, 29, 1659 (in Chinese). (王道林, 韩珊, 黄孝东, 谷峥, 有机化学, 2009, 29, 1659.) (d) Wang, D.; Xu, J.; Han, S.; Gu, Z. Chin. J. Org. Chem. 2008, 28, 2016 (in Chinese). (王道林, 徐姣, 韩珊, 谷峥, 有机化学, 2008, 28, 2016.) (e)Asato, A. E.; Peng, A.; Hossain, M. Z.; Mirzadegan, T.; Bertram, J. J. Med. Chem. 1993, 36, 3137. (f) Becker, D. A.; Ley, J. J.; Echegoyen, L.; Alvarado, R. J. Am. Chem. Soc. 2002, 124, 4678.
[6] (a) Smits, E. C. P.; Setayesh, S.; Anthopoulos, T. D.; Buechel, M.; Nijssen, W.; Coehoorn, R.; Blom, P. W. M.; de Boer, B.; de Leeuw, D. M. Adv. Mater. 2007, 19, 734. (b) Wobkenberg, P. H.; Labram, J. G.; Swiecicki, J.-M.; Parkhomenko, K.; Sredojevic, D.; Gisselbrecht, J.-P.; de Leeuw, D. M.; Bradley, D. D. C.; Djukic, J.-P.; Anthopoulos, T. D. J. Mater. Chem. 2010, 20, 3673. (c) Yamaguchi, Y.; Maruya, Y.; Katagiri, H.; Nakayama, K.-i.; Ohba, Y. Org. Lett. 2012, 14, 2316. (d) Yamaguchi, Y.; Ogawa, K.; Nakayama, K.-i.; Ohba, Y.; Katagiri, H. J. Am. Chem. Soc. 2013, 135, 19095. (e) Yamaguchi, Y.; Takubo, M.; Ogawa, K.; Nakayama, K.-i.; Koganezawa, T.; Katagiri, H. J. Am. Chem. Soc. 2016, 138, 11335. (f) Yao, J.; Cai, Z.; Liu, Z.; Yu, C.; Luo, H.; Yang, Y.; Yang, S.; Zhang, G.; Zhang, D. Macromolecules 2015, 48, 2039. (g) Xin, H.; Ge, C.; Fu, L.; Yang, X.; Gao, X. Chin. J. Org. Chem. 2017, 37, 711 (in Chinese). (辛涵申, 葛从伍, 傅丽娜, 杨笑迪, 高希珂, 有机化学, 2017, 37, 711.)
[7] (a) Umeyama, T.; Watanabe, Y.; Miyata, T.; Imahori, H. Chem. Lett. 2015, 44, 47. (b) Chen, Y.; Zhu, Y.; Yang, D.; Zhao, S.; Zhang, L.; Yang, L.; Wu, J.; Huang, Y.; Xu, Z.; Lu, Z. Chem. Eur. J. 2016, 22, 14527. (c) Puodziukynaite, E.; Wang, H. W.; Lawrence, J.; Wise, A. J.; Russell, T. P.; Barnes, M. D.; Emrick, T. J. Am. Chem. Soc. 2014, 136, 11043.
[8] (a) Nishimura, H.; Ishida, N.; Shimazaki, A.; Wakamiya, A.; Saeki, A.; Scott, L. T.; Murata, Y. J. Am. Chem. Soc. 2015, 137, 15656. (b) Truong, M. A.; Lee, J.; Nakamura, T.; Seo, J. Y.; Jung, M.; Ozaki, M.; Shimazaki, A.; Shioya, N.; Hasegawa, T.; Murata, Y.; Zakeeruddin, S. M.; Grätzel, M.; Murdey, R.; Wakamiya, A. Chem. Eur. J. 2019, 25, 6741.
[9] (a) Asato, A. E.; Liu, R. S. H.; Rao, V. P.; Cai, Y. M. Tetrahedron Lett. 1996, 37, 419. (b) Iftime, G.; Lacroix, P. G.; Nakatani, K.; Razus, A. C. Tetrahedron Lett. 1998, 39, 6853. (c) Lacroix, P. G.; Malfant, I.; Iftime, G.; Razus, A. C.; Nakatani, K.; Delaire, J. A. Chem. Eur. J. 2000, 6, 2599. (d) Coe, B. J.; Harris, J. A.; Asselberghs, I.; Clays, K.; Olbrechts, G.; Persoons, A.; Hupp, J. T.; Johnson, R. C.; Coles, S. J.; Hursthouse, M. B.; Nakatani, K. Adv. Funct. Mater. 2002, 12, 110. (e) Cristian, L.; Sasaki, I.; Lacroix, P. G.; Donnadieu, B.; Asselberghs, I.; Clays, K.; Razus, A. C. Chem. Mater. 2004, 16, 3543. (f) Migalska-Zalas, A.; El kouari, Y.; Touhtouh, S. Opt. Mater. 2012, 34, 1639. (g) Herrmann, R.; Pedersen, B.; Wagner, G.; Youn, J.-H. J. Organomet. Chem. 1998, 571, 261.
[10] (a) Kurotobi, K.; Kim, K. S.; Noh, S. B.; Kim, D.; Osuka, A. Angew. Chem., Int. Ed. 2006, 45, 3944. (b) Wang, F. K.; Lin, T. T.; He, C. B.; Chi, H.; Tang, T.; Lai, Y. H. J. Mater. Chem. 2012, 22, 10448. (c) Ince, M.; Bartelmess, J.; Kiessling, D.; Dirian, K.; Martinez-Diaz, M. V.; Torres, T.; Guldi, D. M. Chem. Sci. 2012, 3, 1472.
[11] (a) Ito, S.; Morita, N. Eur. J. Org. Chem. 2009, 4567. (b) Dong, J.; Zhang, H. Chin. Chem. Lett. 2016, 27, 1097. (c) Xin, H.; Gao, X. ChemPlusChem 2017, 82, 945. (d) Ou, L.; Zhou, Y.; Wu, B.; Zhu, L. Chin. Chem. Lett. 2019, 30, 1903.
[12] Lemal, D. M.; Goldman, G. D. J. Chem. Educ. 1988, 65, 923.
[13] Horowitz, G.; Kouki, F.; Spearman, P.; Fichou, D.; Nogues, C.; Pan, X.; Garnier, F. Adv. Mater. 1996, 8, 242.
[14] (a) Yang, N.; Qiao, X.; Fang, R.; Tao, J.; Hao, J.; Li, H. Acta Chim. Sinica 2016, 74, 335 (in Chinese). (杨宁, 乔小兰, 房忍忍, 陶竞炜, 郝健, 李洪祥, 化学学报, 2016, 74, 335.) (b) Yan, H.; Chen, Z.; Zheng, Y.; Newman, C.; Quinn, J. R.; Dotz, F.; Kastler, M.; Facchetti, A. Nature 2009, 457, 679. (c) Gao, X. K.; Di, C. A.; Hu, Y. B.; Yang, X. D.; Fan, H. Y.; Zhang, F.; Liu, Y. Q.; Li, H. X.; Zhu, D. B. J. Am. Chem. Soc. 2010, 132, 3697. (d) Wang, Y.; Guo, H.; Ling, S.; Arrechea-Marcos, I.; Wang, Y.; López Navarrete, J. T.; Ortiz, R. P.; Guo, X. Angew. Chem., Int. Ed. 2017, 56, 9924.
[15] (a) Jia, T.; Zheng, N.; Cai, W.; Ying, L.; Huang, F. Acta Chim. Sinica 2017, 75, 808 (in Chinese). (贾涛, 郑楠楠, 蔡万清, 应磊, 黄飞, 化学学报, 2017, 75, 808.) (b) Gupta, M.; Yan, D.; Shen, F.; Xu, J.; Zhan, C. Acta Phys.-Chim. Sin. 2019, 35, 496 (in Chinese). (GUPTA Monika, 闫东, 沈福刚, 徐建中, 詹传郎, 物理化学学报, 2019, 35, 496.) (c) Deng, Y.; Peng, A.; Wu, X.; Chen, H.; Huang, H. Acta Phys.-Chim. Sin. 2019, 35, 461 (in Chinese). (邓祎华, 彭爱东, 吴筱曦, 陈华杰, 黄辉, 物理化学学报, 2019, 35, 461.) (d) Zhou, S.; Feng, G.; Xia, D.; Li, C.; Wu, Y.; Li, W. Acta Phys.-Chim. Sin. 2018, 34, 344 (in Chinese). (周士超, 冯贵涛, 夏冬冬, 李诚, 武永刚, 李韦伟, 物理化学学报, 2018, 34, 344.) (e) Ma, Z.; Winands, T.; Liang, N.; Meng, D.; Jiang, W.; Doltsinis, N. L.; Wang, Z. Sci. China Chem. 2020, 63, 208. (f)Liu, J.; Chen, S.; Qian, D.; Gautam, B.; Yang, G.; Zhao, J.; Bergqvist, J.; Zhang, F.; Ma, W.; Ade, H.; Inganäs, O.; Gundogdu, K.; Gao, F.; Yan, H. Nat. Energy 2016, 1, 16089.
[16] Guo, X.; Facchetti, A.; Marks, T. J. Chem. Rev. 2014, 114, 8943.
[17] Xin, H.; Ge, C.; Yang, X.; Gao, H.; Yang, X.; Gao, X. Chem. Sci. 2016, 7, 6701.
[18] (a) Xin, H.; Li, J.; Ge, C.; Yang, X.; Xue, T.; Gao, X. Mater. Chem. Front. 2018, 2, 975. (b) Xin, H.; Ge, C.; Jiao, X.; Yang, X.; Rundel, K.; McNeill, C. R.; Gao, X. Angew. Chem., Int. Ed. 2018, 57, 1322.
[19] Gao, H.; Yang, X.; Xin, H.; Gao, T.; Gong, H.; Gao, X. Chin. J. Org. Chem. 2018, 38, 2680 (in Chinese). (高洪磊, 杨笑迪, 辛涵申, 高铁阵, 龚和贵, 高希珂, 有机化学, 2018, 38, 2680.)
[20] Frisch, M. J.; Trucks, G. W.; Schlegel, H. B.; Scuseria, G. E.; Robb, M. A.; Cheeseman, J. R.; Scalmani, G.; Barone, V.; Petersson, G. A.; Nakatsuji, H.; Li, X.; Caricato, M.; Marenich, A. V.; Bloino, J.; Janesko, B. G.; Gomperts, R.; Mennucci, B.; Hratchian, H. P.; Ortiz, J. V.; Izmaylov, A. F.; Sonnenberg, J. L.; Williams-Young, D.; Ding, F.; Lipparini, F.; Egidi, F.; Goings, J.; Peng, B.; Petrone, A.; Henderson, T.; Ranasinghe, D.; Zakrzewski, V. G.; Gao, J.; Rega, N.; Zheng, G.; Liang, W.; Hada, M.; Ehara, M.; Toyota, K.; Fukuda, R.; Hasegawa, J.; Ishida, M.; Nakajima, T.; Honda, Y.; Kitao, O.; Nakai, H.; Vreven, T.; Throssell, K.; Montgomery, J. A., Jr.; Peralta, J. E.; Ogliaro, F.; Bearpark, M. J.; Heyd, J. J.; Brothers, E. N.; Kudin, K. N.; Staroverov, V. N.; Keith, T. A.; Kobayashi, R.; Normand, J.; Raghavachari, K.; Rendell, A. P.; Burant, J. C.; Iyengar, S. S.; Tomasi, J.; Cossi, M.; Millam, J. M.; Klene, M.; Adamo, C.; Cammi, R.; Ochterski, J. W.; Martin, R. L.; Morokuma, K.; Farkas, O.; Foresman, J. B.; Fox, D. J. Gaussian 16, Gaussian, Inc., Wallingford CT, 2016.
[21] Brown, A. R.; Jarrett, C. P.; deLeeuw, D. M.; Matters, M. Synth. Met. 1997, 88, 37.
[22] (a) Lei, T.; Dou, J.; Pei, J. Adv. Mater. 2012, 24, 6457. (b) Zhang, F.; Hu, Y.; Schuettfort, T.; Di, C. A.; Gao, X.; McNeill, C. R.; Thomsen, L. S.; Mannsfeld, C.; Yuan, W.; Sirringhaus, H.; Zhu, D. J. Am. Chem. Soc. 2013, 135, 2338. |
| [1] |
刘蕊, 孟彬, 胡俊丽, 刘俊. 具有超低LUMO/HOMO能级的稠环芳烃分子★[J]. 化学学报, 2023, 81(10): 1295-1300. |
| [2] |
汪洋, 向焌钧, 葛从伍, 高希珂. 2,6-薁和3,4-丙撑二氧噻吩共聚物的主链结构调控及性质研究★[J]. 化学学报, 2023, 81(10): 1341-1349. |
| [3] |
孙嘉贤, 刘禹廷, 尹志刚, 郑庆东. 基于吸收互补有机半导体本体复合薄膜的高性能柔性光突触晶体管[J]. 化学学报, 2022, 80(7): 936-945. |
| [4] |
刘丽萱, 杨扬, 魏志祥. 手性有机光电功能材料及其圆偏振光发射与探测[J]. 化学学报, 2022, 80(7): 970-992. |
| [5] |
李善武, 朱陈宇杰, 罗尹豪, 张亚茹, 滕汉明, 王宗瑞, 甄永刚. 酰胺与酰亚胺类n型有机半导体材料的研究进展[J]. 化学学报, 2022, 80(12): 1600-1617. |
| [6] |
殷东, 商宏怡, 余文浩, 向仕凯, 胡平, 赵可清, 冯春, 汪必琴. 三唑环修饰的苯并菲二羧酸酯和酰亚胺: 合成、液晶及凝胶性[J]. 化学学报, 2022, 80(10): 1376-1384. |
| [7] |
段超, 张建伟, 向焌钧, 杨笑迪, 高希珂. 硼氮杂薁基[4]螺烯的设计合成及性质研究※[J]. 化学学报, 2022, 80(1): 29-36. |
| [8] |
胡鑫明, 钟春晓, 李晓艳, 贾雄, 魏颖, 解令海. 环戊并二噻吩衍生物的合成及其应用[J]. 化学学报, 2021, 79(8): 953-966. |
| [9] |
王金凤, 李振. 聚集态分子排列对光电性能的影响[J]. 化学学报, 2021, 79(5): 575-587. |
| [10] |
朱雪敏, 白小燕, 王海峰, 胡平, 汪必琴, 赵可清. 苯并䓛盘状液晶: 合成、柱状相和光物理性质[J]. 化学学报, 2021, 79(12): 1486-1493. |
| [11] |
任江波, 王蕾, 郭锐, 唐永和, 周红梅, 林伟英. 一种基于萘酰亚胺的检测细胞内pH值的荧光探针及其生物成像应用[J]. 化学学报, 2021, 79(1): 87-92. |
| [12] |
边洋爽, 刘凯, 郭云龙, 刘云圻. 功能性可拉伸有机电子器件的研究进展[J]. 化学学报, 2020, 78(9): 848-864. |
| [13] |
胡瑜辉, 武文林, 于立扬, 骆开均, 徐小鹏, 李瑛, 彭强. 基于吡咯并吡咯二酮核心的苝二酰亚胺类受体分子的合成及光伏性能[J]. 化学学报, 2020, 78(11): 1246-1254. |
| [14] |
张衡, 牟学清, 陈弓, 何刚. 铜催化苯甲酰亚胺高烯丙酯的分子内胺化全氟烷基化反应[J]. 化学学报, 2019, 77(9): 884-888. |
| [15] |
薄一凡, 刘玉玉, 常永正, 李银祥, 张效霏, 宋春元, 许卫锋, 曹洪涛, 黄维. 环状芴基张力半导体拉曼光谱理论与实验研究[J]. 化学学报, 2019, 77(5): 442-446. |
|