化学学报 ›› 2021, Vol. 79 ›› Issue (10): 1286-1292.DOI: 10.6023/A21060280 上一篇 下一篇
研究论文
投稿日期:2021-06-17
发布日期:2021-07-14
通讯作者:
郑淞生, 王兆林
基金资助:
Riyi Chen, Songsheng Zheng( ), Zhibin Lin, Yunquan Liu, Zhaolin Wang(
), Zhibin Lin, Yunquan Liu, Zhaolin Wang( )
)
Received:2021-06-17
Published:2021-07-14
Contact:
Songsheng Zheng, Zhaolin Wang
Supported by:文章分享

氨(NH3)是一种无碳氢载体, 比氢更易储运, 且体积能量密度更高, 因此直接氨燃料电池(DAFC)的研究具有重要的理论意义和实际价值. 本工作研究PtIr/C阳极催化剂在不同工作温度下电催化活性及其对DAFC性能的影响, 并探究了所用阴离子交换膜的渗氨量与DAFC性能的相关性. 结果表明, 从25~80 ℃, 基于PtIr/C阳极催化剂的DAFC在80 ℃下获得最优性能, 其开路电压(OCV) 0.50 V, 峰值功率密度3.2 mW•cm-2, 可归功于Pt-Ir合金的协同作用和升温提高了催化活性. 不同温度下在DAFC阴极尾气中均检测到氨, 且氨含量随温度升高而上升, 致使阴极Pt/C催化剂毒化, 从而使DAFC的开路电压和功率密度下降.
陈日懿, 郑淞生, 林志彬, 刘运权, 王兆林. 基于PtIr/C阳极催化剂的直接氨燃料电池性能研究[J]. 化学学报, 2021, 79(10): 1286-1292.
Riyi Chen, Songsheng Zheng, Zhibin Lin, Yunquan Liu, Zhaolin Wang. Performance Study of Direct Ammonia Fuel Cell Based on PtIr/C Anode Electrocatalyst[J]. Acta Chimica Sinica, 2021, 79(10): 1286-1292.
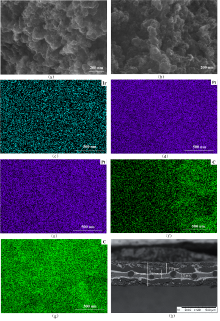



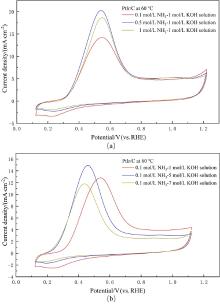
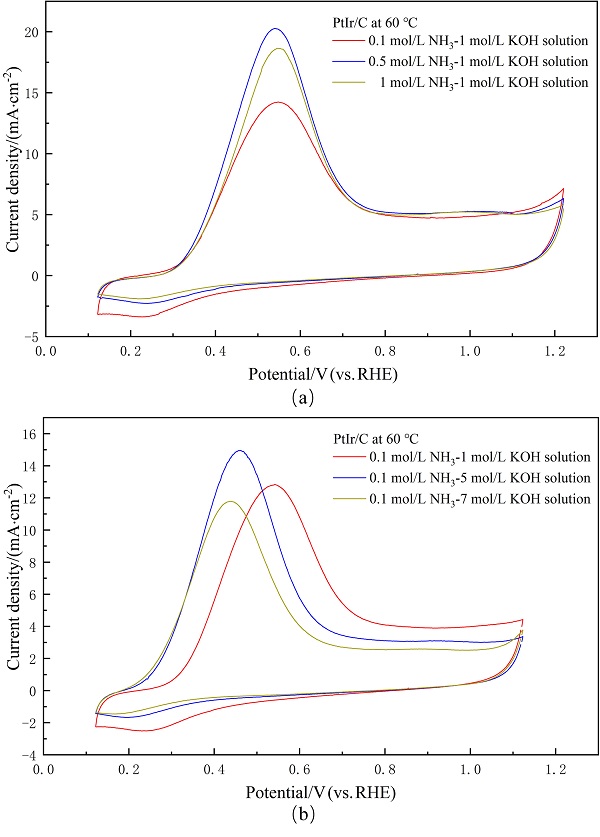
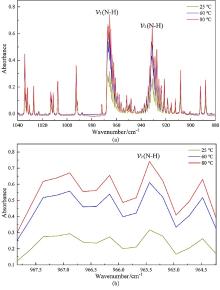

| [1] |
Jiang, T.; Huang, S.; Yang, J. J. Clean Prod. 2019, 240.
|
| [2] |
Stott, R.; Arulkumaran, S.; Gilmore, I.; Godlee, F.; Page, L. The Lancet 2019, 393.
|
| [3] |
Tian, L.; Zhang, W.; Xie, Z.; Peng, K.; Ma, Q.; Xu, Q.; Pasupathi, S.; Su, H. Acta Phys.-Chim. Sin. 2020, 37, 2009049. (in Chinese)
|
|
(田立亮, 张玮琦, 解政, 彭凯, 马强, 徐谦, Sivakumar, Pasupathi, 苏华能, 物理化学学报, 2021, 37, 2009049.)
|
|
| [4] |
Lin, J.-Y.; Shao, L.; Si, F.-Z.; Fu, X.-Z.; Luo, J.-L. Int. J. Hydrogen Energy 2018, 43, 19704.
doi: 10.1016/j.ijhydene.2018.08.204 |
| [5] |
Ostroverkh, A.; Johánek, V.; Dubau, M.; Kúš, P.; Khalakhan, I.; Šmíd, B.; Fiala, R.; Václavů, M.; Ostroverkh, Y.; Matolín, V. Int. J. Hydrogen Energy 2019, 44, 19344.
doi: 10.1016/j.ijhydene.2018.12.206 |
| [6] |
Charradi, K.; Ahmed, Z.; Cid, R. E.; Aranda, P.; Ruiz-Hitzky, E.; Ocon, P.; Chtourou, R. Int. J. Hydrogen Energy 2019, 44, 10666.
doi: 10.1016/j.ijhydene.2019.02.183 |
| [7] |
Abbas, N.; Qin, X.; Ali, S.; Zhu, G.; Lu, J.; Alam, F. e.; Wattoo, A. G.; Zeng, X.; Gu, K.; Tang, J. J. Eur. Ceram. Soc. 2020, 40, 3338.
doi: 10.1016/j.jeurceramsoc.2020.02.033 |
| [8] |
Feng, G.; Kuang, Y.; Li, P.; Han, N.; Sun, M.; Zhang, G.; Sun, X. Adv. Sci. (Weinh) 2017, 4, 1600179.
|
| [9] |
Abbasi, R.; Setzler, B. P.; Wang, J.; Zhao, Y.; Wang, T.; Gottesfeld, S.; Yan, Y. Curr. Opin. Electrochem. 2020, 21, 335.
|
| [10] |
Li, Q.; Xu, H.; Tong, Y.; Li, G. Acta Chim. Sinica 2017, 75, 193. (in Chinese)
doi: 10.6023/A16070337 |
|
(李奇, 许瀚, 童叶翔, 李高仁, 化学学报, 2017, 75, 193.)
doi: 10.6023/A16070337 |
|
| [11] |
Jin, X.; Wei, Q.; Huang, H.; Wang, M.; Huang, Y. Chin. J. Chem. 2012, 30, 2805.
doi: 10.1002/cjoc.v30.12 |
| [12] |
Mott, J. Ammonia = Hydrogen 2.0 Conference: panel discussion recap. Ammonia Energy Assoc September 12, 2019. https://www.ammoniaenergy.org/articles/ammonia-hydrogen-2-0-conference-panel-discussionrecap/
|
| [13] |
Giddey, S.; Badwal, S. P. S.; Munnings, C.; Dolan, M. ACS Sustain. Chem. Eng. 2017, 5, 10231.
doi: 10.1021/acssuschemeng.7b02219 |
| [14] |
Zhan, S.; Zhang, F. Acta Chim. Sinica 2021, 79, 146. (in Chinese)
doi: 10.6023/A20090412 |
|
(詹溯, 章福祥, 化学学报, 2021, 79, 146.)
doi: 10.6023/A20090412 |
|
| [15] |
Feng, S.; Gao, W.; Cao, H.; Guo, J.; Chen, P. Acta Chim. Sinica 2020, 78, 916. (in Chinese)
doi: 10.6023/A20060207 |
|
(冯圣, 高文波, 曹湖军, 郭建平, 陈萍, 化学学报, 2020, 78, 916.)
doi: 10.6023/A20060207 |
|
| [16] |
Wan, Z.; Tao, Y.; Shao, J.; Zhang, Y.; You, H. Energy Convers. Manage. 2021, 228.
|
| [17] |
Afif, A.; Radenahmad, N.; Cheok, Q.; Shams, S.; Kim, J. H.; Azad, A. K. Renew. Sust. Energ. Rev. 2016, 60, 822.
doi: 10.1016/j.rser.2016.01.120 |
| [18] |
Halseid, R.; Vie, P. J. S.; Tunold, R. J. Power Sources 2006, 154, 343.
doi: 10.1016/j.jpowsour.2005.10.011 |
| [19] |
Tewari, A.; Sambhy, V.; Urquidi Macdonald, M.; Sen, A. J. Power Sources 2006, 153, 1.
doi: 10.1016/j.jpowsour.2005.03.192 |
| [20] |
Zhao, Y.; Setzler, B. P.; Wang, J.; Nash, J.; Wang, T.; Xu, B.; Yan, Y. Joule 2019, 3, 2472.
doi: 10.1016/j.joule.2019.07.005 |
| [21] |
Gottesfeld, S. J. Electrochem. Soc. 2018, 165, J3405.
doi: 10.1149/2.0431815jes |
| [22] |
Li, Y.; Pillai, H. S.; Wang, T.; Hwang, S.; Zhao, Y.; Qiao, Z.; Mu, Q.; Karakalos, S.; Chen, M.; Yang, J.; Su, D.; Xin, H.; Yan, Y.; Wu, G. Energy Environ. Sci. 2021, 14, 1449.
doi: 10.1039/D0EE03351K |
| [23] |
Siddiqui, O.; Dincer, I. Fuel Cells 2018, 18, 379.
doi: 10.1002/fuce.v18.4 |
| [24] |
Abbasi, R.; Setzler, B. P.; Lin, S.; Wang, J.; Zhao, Y.; Xu, H.; Pivovar, B.; Tian, B.; Chen, X.; Wu, G.; Yan, Y. Adv. Mater. 2019, 31, e1805876.
|
| [25] |
Kamarudin, M. Z. F.; Kamarudin, S. K.; Masdar, M. S.; Daud, W. R. W. Int. J. Hydrogen Energy 2013, 38, 9438.
doi: 10.1016/j.ijhydene.2012.07.059 |
| [26] |
Takahashi, S.; Mashio, T.; Horibe, N.; Akizuki, K.; Ohma, A. ChemElectroChem 2015, 2, 1560.
doi: 10.1002/celc.v2.10 |
| [27] |
Song, L.; Liang, Z.; Ma, Z.; Zhang, Y.; Chen, J.; Adzic, R. R.; Wang, J. X. J. Electrochem. Soc. 2018, 165, J3095.
doi: 10.1149/2.0181815jes |
| [28] |
Silva, J. C. M.; Ntais, S.; Teixeira-Neto, É.; Spinacé, E. V.; Cui, X.; Neto, A. O.; Baranova, E. A. Int. J. Hydrogen Energy 2017, 42, 193.
doi: 10.1016/j.ijhydene.2016.09.135 |
| [29] |
Suzuki, S.; Muroyama, H.; Matsui, T.; Eguchi, K. J. Power Sources 2012, 208, 257.
doi: 10.1016/j.jpowsour.2012.02.043 |
| [30] |
de Vooys, A. C. A.; Koper, M. T. M.; van Santen, R. A.; van Veen, J. A. R. J. Electroanal. Chem. 2001, 506, 127.
doi: 10.1016/S0022-0728(01)00491-0 |
| [31] |
Assumpção, M. H. M. T.; da Silva, S. G.; de Souza, R. F. B.; Buzzo, G. S.; Spinacé, E. V.; Neto, A. O.; Silva, J. C. M. Int. J. Hydrogen Energy 2014, 39, 5148.
doi: 10.1016/j.ijhydene.2014.01.053 |
| [32] |
Sacré, N.; Duca, M.; Garbarino, S.; Imbeault, R.; Wang, A.; Hadj Youssef, A.; Galipaud, J.; Hufnagel, G.; Ruediger, A.; Roué, L.; Guay, D. ACS Catal. 2018, 8, 2508.
doi: 10.1021/acscatal.7b02942 |
| [33] |
Adli, N. M.; Zhang, H.; Mukherjee, S.; Wu, G. J. Electrochem. Soc. 2018, 165, J3130.
doi: 10.1149/2.0191815jes |
| [34] |
Chen, R.; Zheng, S.; Yao, Y.; Lin, Z.; Ouyang, W.; Zhuo, L.; Wang, Z. Int. J. Hydrogen Energy 2021, 46, 27749.
doi: 10.1016/j.ijhydene.2021.06.001 |
| [35] |
Kontou, S.; Stergiopoulos, V.; Song, S.; Tsiakaras, P. J. Power Sources 2007, 171, 1.
doi: 10.1016/j.jpowsour.2006.10.009 |
| [36] |
Hren, M.; Božič, M.; Fakin, D.; Kleinschek, K. S.; Gorgieva, S. Sustain. Energ. Fuels 2021, 5, 604.
doi: 10.1039/D0SE01373K |
| [37] |
Hagesteijn, K. F. L.; Jiang, S.; Ladewig, B. P. J. Mater. Sci. 2018, 53, 11131.
doi: 10.1007/s10853-018-2409-y |
| [38] |
Qi, Z.; Kaufman, A. J. Power Sources 2002, 110, 177.
doi: 10.1016/S0378-7753(02)00268-9 |
| [39] |
Giovannozzi, A. M.; Pennecchi, F.; Muller, P.; Balma Tivola, P.; Roncari, S.; Rossi, A. M. Anal. Bioanal. Chem. 2015, 407, 8423.
doi: 10.1007/s00216-015-9030-6 pmid: 26377936 |
| [1] | 李雅宁, 王晓艳, 唐勇. 自由基聚合的立体选择性调控★[J]. 化学学报, 2024, 82(2): 213-225. |
| [2] | 刘士琨, 邓程维, 姬峰, 闵宇霖, 李和兴. 高温质子交换膜燃料电池中阴极双催化层孔结构的设计研究★[J]. 化学学报, 2023, 81(9): 1135-1141. |
| [3] | 崔国庆, 胡溢玚, 娄颖洁, 周明霞, 李宇明, 王雅君, 姜桂元, 徐春明. CO2加氢制醇类催化剂的设计制备及性能研究进展[J]. 化学学报, 2023, 81(8): 1081-1100. |
| [4] | 付信朴, 王秀玲, 王伟伟, 司锐, 贾春江. 团簇Au/CeO2的制备及其催化CO氧化反应构效关系的研究★[J]. 化学学报, 2023, 81(8): 874-883. |
| [5] | 刘建川, 李翠艳, 刘耀祖, 王钰杰, 方千荣. 高稳定二维联咔唑sp2碳共轭共价有机框架材料用于高效电催化氧还原★[J]. 化学学报, 2023, 81(8): 884-890. |
| [6] | 赵天成, 蒋鸿宇, 张琨, 徐一帆, 康欣悦, 胥鉴宸, 周旭峰, 陈培宁, 彭慧胜. 基于环烷烃/乙醇混合碳源高性能碳纳米管纤维的连续化制备[J]. 化学学报, 2023, 81(6): 565-571. |
| [7] | 王子豪, 陈敏, 陈昶乐. 不对称α-二亚胺镍催化制备聚烯烃弹性体★[J]. 化学学报, 2023, 81(6): 559-564. |
| [8] | 刘露杰, 张建, 王亮, 肖丰收. 生物质基多元醇的多相催化选择性氢解★[J]. 化学学报, 2023, 81(5): 533-547. |
| [9] | 徐斌, 韦秀芝, 孙江敏, 刘建国, 马隆龙. 原位合成氮掺杂石墨烯负载钯纳米颗粒用于催化香兰素高选择性加氢反应[J]. 化学学报, 2023, 81(3): 239-245. |
| [10] | 刘健, 欧金花, 李泽平, 蒋婧怡, 梁荣涛, 张文杰, 刘开建, 韩瑜. 金属-有机骨架衍生的Co单原子高效催化硝基芳烃氢化还原[J]. 化学学报, 2023, 81(12): 1701-1707. |
| [11] | 刘金晶, 杨娜, 李莉, 魏子栋. 铂活性位空间结构调控氧还原机理的理论研究★[J]. 化学学报, 2023, 81(11): 1478-1485. |
| [12] | 杨贯文, 伍广朋. 模块化双功能有机硼氮和硼磷催化体系的设计及其催化转化★[J]. 化学学报, 2023, 81(11): 1551-1565. |
| [13] | 王庆鑫, 崔勇, 李蕴琪, 卢善富, 相艳. Fe-N-C阴极催化层离聚物可控热解对膜电极性能与稳定性的影响研究★[J]. 化学学报, 2023, 81(10): 1350-1356. |
| [14] | 李波, 周海燕, 马海燕, 黄吉玲. 亚乙基桥联双茚锆、铪配合物的合成及催化丙烯选择性齐聚研究: 茚环3-位取代基的影响[J]. 化学学报, 2023, 81(10): 1280-1294. |
| [15] | 陈治平, 孟永乐, 芦静, 周文武, 杨志远, 周安宁. Fe@Si/S-34催化剂的制备及其合成气制烯烃性能[J]. 化学学报, 2023, 81(1): 14-19. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||